How do I approach solving problems
like #1-5? These problems require factual knowledge and
an ability to reason. Pretend you are a doctor and
patient has certain symptoms (the facts presented) and
you must call upon your knowledge to provide a diagnosis
(solution). Here
is a step-by-step analysis (diagnosis) of
#2
PS7 from F2000. I recommend
that you do question 6 first. it will give you some
background. Vladimir
Vasilovich Markovnikov (1838-1904)

|
1. Alkene A reacts with chlorine to form meso B. Ozonolysis of A forms a single compound C. Hydrogenation of A liberates 27.3 kcal/mol of heat to form a normal chain alkane D with an estimated ΔHfo of -59.8 kcal/mol. What are the structures A-D? Explain and illustrate. |
|
2. Compound A is optically active and it absorbs two equivalents of H2 to form optically active B (C10H20). Treatment of A with warm, concentrated KMnO4 forms (R)-2-methylsuccinic acid (tartaric acid = 2,3-dihydroxysuccinic acid). What are the structures of A and B? Explain and illustrate. |
|
3. Hydroboration-peroxide oxidation is said to be an anti-Markovnikov addition of water to an unsymmetrically substituted double bond. Yet, hydroboration of an unsymmetrically substituted double bond is a Markovnikov addition of borane. Explain and illustrate. |
|
4. Hydroboration of (Z,Z)-1,4-cyclooctadiene at room temperature gives two boranes, A and B, both with the formula C8H15B. Alkaline peroxide oxidation of A gives a 1,4-cycloctane diol (di-alcohol) while B gives the 1,5-isomer. When a mixture of A and B, or either one of them on their own, is heated, cooled and oxidized, B dominates over A. a) What is the stereochemistry of each diol? b) Explain and illustrate the conditions under which A and B are formed vs. B alone. A reaction co-ordinate diagram would be helpful. |
|
5. 2,6-Octadiene A (C8H14) of unknown stereochemistry reacts with catalytic OsO4/H2O2 to form two racemic tetraols (four hydroxyl groups). Two geometrically isomeric 2,6-octadienes, B and C, each afford a racemic and meso tetraol. Hydrogenaton of B liberates 2.0 kcal/mol more heat than C. What are the structures of A-C and of the tetraols? The tetraols are best illustrated as Fischer projections. |
|
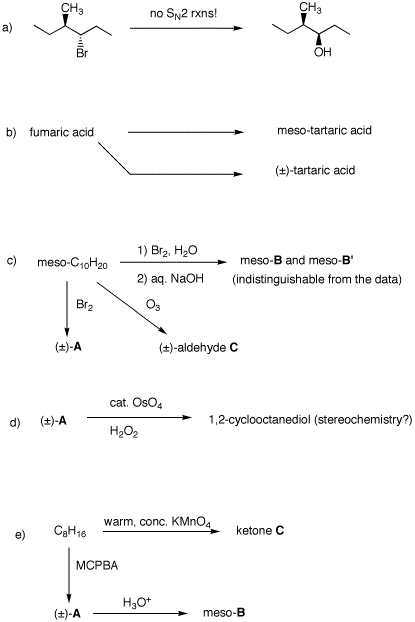
6. Provide solutions to each of the following problems by supplying reagents, structures, and brief explanations.
|