|
In our lab we have explored the application of non-linear spectroscopic techniques as tools for probing molecular dynamics, primarily in gas-phase systems. In particular, we have used Degenerate Four-Wave Mixing (DFWM) to perform such studies. The basic principle in DFWM is the generation of coherences in an ensemble of molecules by the application of strong laser fields. These coherences have the unique capability of Bragg-scattering light, which in turn creates a signal beam. In one of its traditional implementations, the forward-box geometry, three coherent waves of light of the same frequency travel through the major diagonal of a box. Two of these fields would generate a grating (coherence), and the third field would be scattered from this grating, generating a fourth beam. The small angles used to cross these beams (typically ~1°) ensure a broad overlapping area, which increases the efficiency of the scattering process. The direction of the DFWM signal is constrained by momentum conservation (phase matching condition) and would travel through the remaining diagonal of the box. Its frequency would be determined by energy conservation, which requires the fourth beam to have the same color as the others. The intensity of this signal depends, among other factors, on whether or not the common color of the input fields matches a transition within the probed system. This resonance condition generates a "background-free" response, which tremendously increases the sensitivity of DFWM. Another advantage of DFWM is that its polarization properties can be exploited to simplify complex spectra in ways that have no analogy to its linear counterparts. In our lab we have developed new polarization schemes that permit the identification of rovibronic features based on their change in total angular momentum (ΔJ) upon excitation. This means that DFWM provides an easy way to selectively suppress and identify P, Q, and R rovibronic bands. This characteristic greatly simplifies the analysis of electronic spectra in large polyatomic systems, where the small inertial constants produce huge numbers of overlapping transitions. One of the processes that our group has been investigating is the intramolecular proton transfer. This mechanism plays a crucial role in a large number of biological reactions. The model system to investigate this phenomenon is malonaldehyde, which is the smallest organic molecule where the intramolecular proton transfer can be detected. Our studies have probed the change in proton-transfer dynamics upon excitation to the π* ← n state. This electronic transition cannot be easily explored by traditional spectroscopic techniques, in part because the short lifetime observed in this state (less than 5 ps), precludes the possibility of spontaneous or induced emission. By applying DFWM to study this state, we have been able to identify a decrease in the proton transfer rate upon excitation. These experimental results suggest an increase in the barrier height for the proton transfer, which has also been predicted in recent ab initio calculations. The DFWM signal dependence on high powers of the transition moments and number density manifests by the suppression of weak transitions and the enhancement of strong transitions. This particular characteristic of DFWM has allowed us to easily identify vibronic progressions in the excited electronic state of malonaldehyde, which were obscured in the linear absorption scan of this transition. Another similar system to malonaldehyde that offers a tractable system to study the intramolecular proton transfer dynamics is tropolone. Even though this molecule also displays a symmetric double well like malonaldehyde does, its larger size produces a large degree of spectroscopic congestion that has prevented a detailed analysis of its spectrum. We have focused our efforts on the π* ← π excitation of tropolone and by exploiting our newly developed polarization arrangements we have successfully extracted rotational constants for the first two vibronic bands of this transition. These inertial constants give us an idea of structural changes in tropolone during the proton transfer process and indicate a favored direction in molecular displacements during the hydrogen migration. 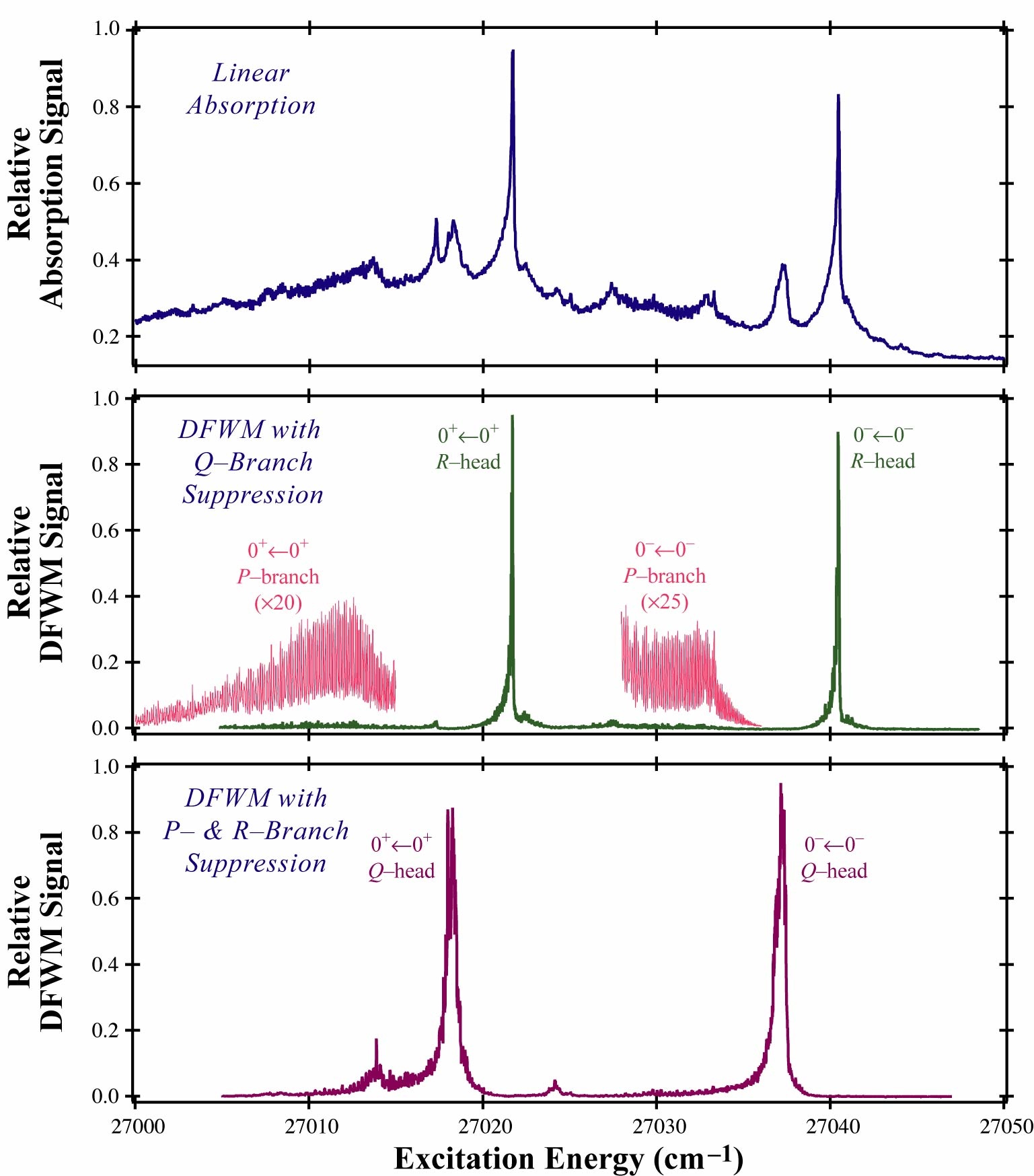
Degenerate Four-Wave Mixing spectrum of A1B2 tropolone |