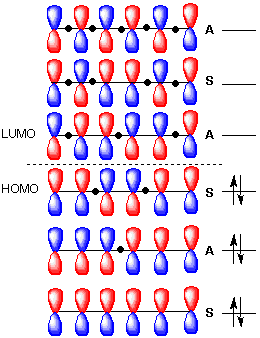
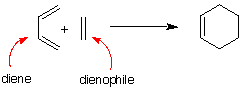
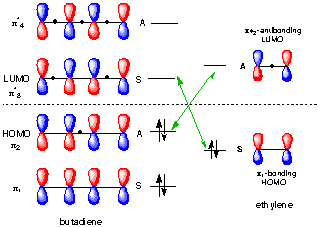
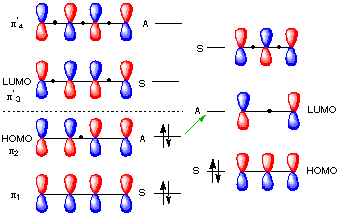
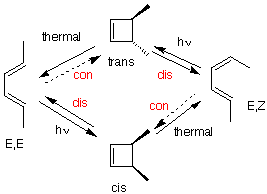
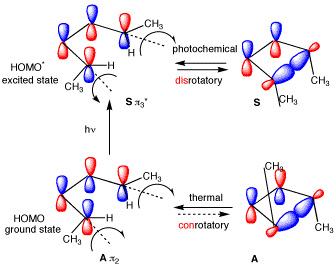
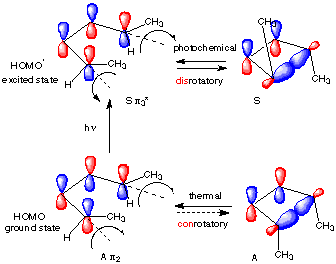
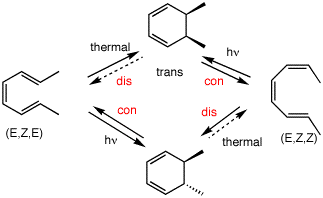
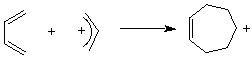Figure 1 shows two isolated p-atomic orbitals (AO) of equal energy. When the wave functions of these two orbitals are added to and subtracted from one another, two new molecular orbitals (MOs) are formed. Note the emphasis on two. The same number of MOs are formed from the same number of AOs. One MO ---namely π1 -- is bonding and lower in energy than the parent AOs while π2* is antibonding and higher energy than the p-AOs. These two MOs represent the pi-bond in ethylene. The two paired electrons of the π-bond are placed in the bonding MO, which is the Highest Occupied Molecular Orbital (HOMO). The antibonding MO is the Lowest Unoccupied Molecular Orbital (LUMO) and contains no electrons. The HOMO and LUMO are called frontier MOs (FMOs).
Fig.1