EXAM 3
CHEMISTRY 220
Friday, April 26, 2013
NAME (print): ________________________________________________________
TA:_________________ Sect. Day:_________________ Sect. Time:_____________
Fill in the information above. Place your name on the top right of subsequent pages.
Take a few moments to look over the exam. Answer each question on the exam paper.
No calculators, electronic devices, earbuds, texts or notes. You may use molecular models. Important clues and structures are in bold. There is a Periodic Table on pg. 9. Do all preliminary drawing or computations on the work sheets at the end of the exam. The work sheets will not be graded. You may detach the work sheets from the exam.
The exam is 55 minutes.
STOP writing and hand in your exam when you are asked to do so.
Remember: Neatness is to your advantage.
1. (20 pts) Reactions I (3 of 4) ______
2. (20 pts) Reactions II (2 of 3) ______
3. (20 pts) Mechanisms (2 of 4) ______
4. (20 pts) Structure (1 of 2) ______
5. (20 pts) Synthesis ______
__________________________________________________
Total (100 pts)
1) Reactions: (20 pts.) Complete 3 of 4 of the following problems. If you do all four, clearly cross out the one you do not want graded. Pay particular attention to stereochemistry and well-defined drawings. Explain briefly.
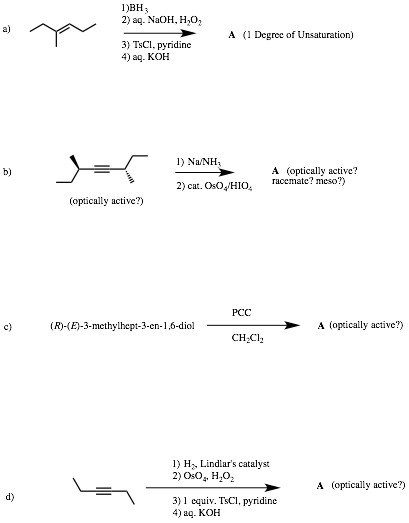
2) Reactions II: (20 pts.) Complete 2 of 3 of the following problems. Identify the unknown structures. No mechanisms required! If you do all three, clearly cross out the one you do not want graded. Explain briefly. [!!!!! signifies important information!]
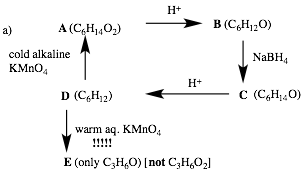
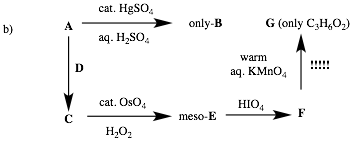
Écontinued
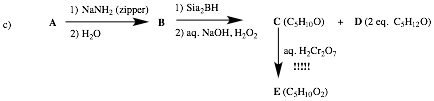
3) Mechanisms: (20 pts.) Complete 2 of 4 of the following questions. If you do 3 or 4, clearly cross out the one(s)
you do not want graded.
a) Provide a mechanism (arrow-pushing) for the oxidation of acetaldehyde to acetic acid using aqueous chlorine (StevensŐ oxidation, PS9).
b) Show a method for the preparation of CD3SCD2H from CD3S(O)CD3 (dimethyl sulfoxide – d6). [Swern oxidation; class 4/17]
Écontinued
c) Anhydrous
chromium (VI) reagents (CollinsŐ reagent or PCC) are often used to oxidize primary
alcohols to aldehydes. Often esters can be formed, e.g., ethanol ---- >
ethyl acetate. Explain and illustrate.
d) The conversion of 2-butene to acetaldehyde by ozonolysis followed by treatment with dimethyl sulfide is a 4-electron oxidation. The same transformation can be accomplished with cat. OsO4 and periodic acid. i) How many equivalents of periodic acid are required? ii) Explain and illustrate using your knowledge of oxidation levels.
3) Structure: (20 pts.) Complete 1 of 2 of the following problems. If you do both of them, clearly cross out the one you do not want graded. Explain briefly.
a) An optically active ester A (C10H20O2) reacts with excess LiAlH4 to provide (±)-B (C5H12O). Compound A reacts with 2 equiv. of Grignard reagent C (RMgBr) to form (S)-B and (R)-D (C7H16O). Provide the structures A-D. Explain briefly. [Recall PS9.]
b) Compound A undergoes a two-electron reduction with reagent B to afford C. Compound C reacts with cat. OsO4/H2O2
to yield D. Treatment of D with one equivalent of
TsCl/pyridine (E)
followed by aqueous KOH affords meso-F (C6H12O).
Provide the structures A-F.
Explain briefly.
5). Synthesis:
(20 pts.) Design a synthesis of the ketone, 4-octanone, using 1-butene as your
only source of carbon (4 + 4 = 8 !!!!!). All inorganic or carbon-bearing reagents
are available to you. [You will most likely need some reactions from previous
chapters.]
Periodic Table
Work Sheet
Work Sheet
Work Sheet