EXAM 3
CHEMISTRY 220
Friday, April 26, 2013
Solutions
NAME (print): ________________________________________________________
TA:_________________ Sect. Day:_________________ Sect. Time:_____________
Fill in the information above. Place your name on the top right of subsequent pages.
Take a few moments to look over the exam. Answer each question on the exam paper.
No calculators, electronic devices, earbuds, texts or notes. You may use molecular models. Important clues and structures are in bold. There is a Periodic Table on pg. 9. Do all preliminary drawing or computations on the work sheets at the end of the exam. The work sheets will not be graded. You may detach the work sheets from the exam.
The exam is 55 minutes.
STOP writing and hand in your exam when you are asked to do so.
Remember: Neatness is to your advantage.
1. (20 pts) Reactions I (3 of 4) ______
2. (20 pts) Reactions II (2 of 3) ______
3. (20 pts) Mechanisms (2 of 4) ______
4. (20 pts) Structure (1 of 2) ______
5. (20 pts) Synthesis ______
__________________________________________________
Total (100 pts)
1) Reactions: (20 pts.) Complete 3 of 4 of the following problems. If you do all four, clearly cross out the one you do not want graded. Pay particular attention to stereochemistry and well-defined drawings. Explain briefly.
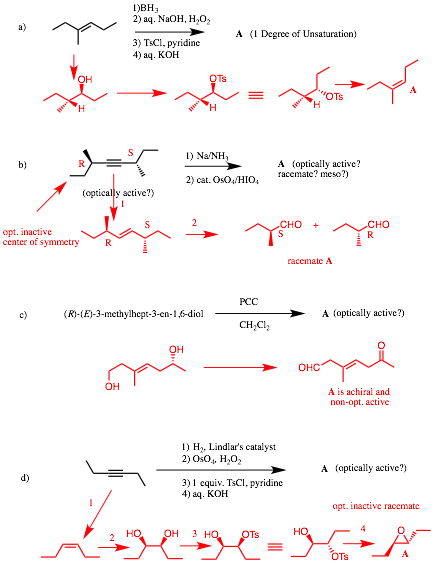
2) Reactions II: (20 pts.) Complete 2 of 3 of the following problems. Identify the unknown structures. No mechanisms required! If you do all three, clearly cross out the one you do not want graded. Explain briefly. [!!!!! signifies important information!]
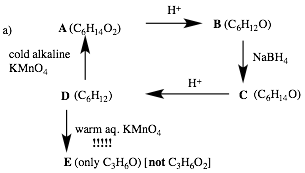
If E were CH3CH2CHO (propionaldehyde) --- as
most people suggested --- it would be oxidized to CH3CH2CO2H
(propionic acid, C3H6O2). E
is acetone ONLY. So D is
tetramethylethylene (2,3-dimethyl-2-butene). COLD permanganate adds 2 OH groups
to form A (2,3-dimethylbutan-2,3-diol, pinacol). Acid effects pinacol rearrangement to
form B (pinacolone,
3,3-dimethyl-2-butanone). Reduction of B
forms 3,3-dimethyl-2-butanol C. Acid
forms a secondary carbocation of B
with methyl migration. Loss of a
proton generates D. Review the
pinacol rearrangement discussed in class.
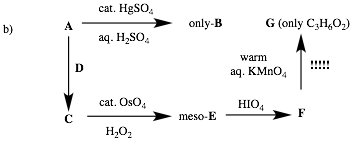
meso-E indicates a 1,2-diol formed by
osmylation of a (Z)-double bond of C. The reaction conditions
of A -> B indicates that A is an alkyne and it is also symmetrical. A single compound B is formed which is a ketone.
D must be a Lindlar reduction
to C that is not only (Z)- but symmetrically substituted with
the same two groups. Since G is
propionic acid formed by the oxidation of F,
itself formed by periodic acid cleavage of meso-E, which is meso-hexan-3,4-diol. F is CH3CH2CHO. C is (Z)-3-hexene. A is
3-hexyne. B is 3-hexanone.
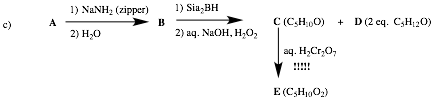
C has one D.U. and it is
oxidized to a C5 carboxylic acid of unknown structure E. C
is the associated aldehyde. B is a
terminal alkyne given the conditions to form aldehyde C. The zipper reaction says that A is an internal alkyne, which can only be 2-pentyne. Therefore, C is pentanal (CH3(CH2)
3CHO and E is pentanoic acid.
What is D? It is “Sia”, secondary
isoamyl alcohol, 3-methyl-2-butanol.
3) Mechanisms: (20 pts.) Complete 2 of 4 of the following questions. If you do 3 or 4, clearly cross out the one(s)
you do not want graded.
a) Provide a mechanism (arrow-pushing) for the oxidation of acetaldehyde to acetic acid using aqueous chlorine (Stevens’ oxidation, PS9).
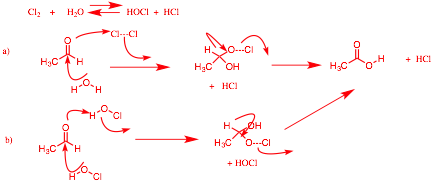
b) Show a method for the preparation of CD3SCD2H from CD3S(O)CD3 (dimethyl sulfoxide – d6). [Swern oxidation; class 4/17]
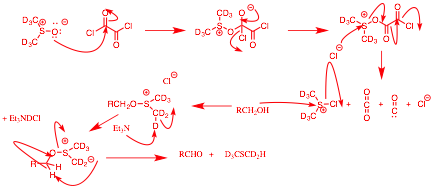
…continued
c) Anhydrous
chromium (VI) reagents (Collins’ reagent or PCC) are often used to oxidize primary
alcohols to aldehydes. Often esters can be formed, e.g., ethanol ---- >
ethyl acetate. Explain and illustrate.
As ethanol is being oxidized to
acetaldehyde in the absence of water, unreacted alcohol can fill the role of
water and add to the aldehyde to form a hemiacetal (shown). As far as the oxidant is concerned this
is an alcohol and it is oxidized to form the carbonyl group of an ester. In this case, ethyl
acetate.
![]()
d) The conversion of 2-butene to acetaldehyde by ozonolysis followed by treatment with dimethyl sulfide is a 4-electron oxidation. The same transformation can be accomplished with cat. OsO4 and periodic acid. i) How many equivalents of periodic acid are required? ii) Explain and illustrate using your knowledge of oxidation levels.
Given the first sentence, cat. OsO4
and periodic acid gets to the same place from the same starting material. This route must also be a 4-electron
oxidation. OsO4 oxidizes 2-butene to the diol and it is an equiv. of
HIO4 that recycles Os(VI) to Os(VIII). The
cleavage of the diol to two moles of acetaldehyde requires a second equiv. of
HIO4.
3) Structure: (20 pts.) Complete 1 of 2 of the following problems. If you do both of them, clearly cross out the one you do not want graded. Explain briefly.
a) An optically active ester A (C10H20O2) reacts with excess LiAlH4 to provide (Ī)-B (C5H12O). Compound A reacts with 2 equiv. of Grignard reagent C (RMgBr) to form (S)-B and (R)-D (C7H16O). Provide the structures A-D. Explain briefly. [Recall PS9.] A clone of PS9 #2. Since B is C5, the acid and the alcohol portion of the ester must both be C5 and form the same C5 alcohol on reduction with LiAlH4. There is only one chiral arrangement of a C5 alcohol, which must be primary. (Reduction of an ester can only give a primary alcohol from the acid portion of the ester). Grignard addition occurs twice to give a C7 compound D. Thus, C is CH3MgBr. Also, (S)-B is lost in this reaction. The (R)-chirality of the ester must be in the carboxylic acid portion.
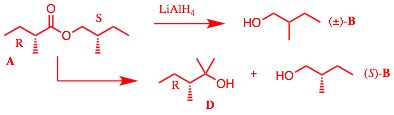
b) Compound A undergoes a two-electron reduction with reagent B to afford C. Compound C reacts with cat. OsO4/H2O2
to yield D. Treatment of D with one equivalent of
TsCl/pyridine (E) followed by
aqueous KOH affords meso-F (C6H12O).
Provide the structures A-F.
Explain briefly. OsO4/H2O2 forms a 1,2-diol D from an alkene C. A two
electron reduction suggests that A
is an alkyne. The monotosylate (E) of the diol undergoes SN2
displacement (single inversion) to afford the meso-epoxide F. Therefore, with a single inversion, C is (E)-2-hexene and the
diol is (Ī)-3,4-hexanediol. A is
3-hexyne and B is Na/NH3.
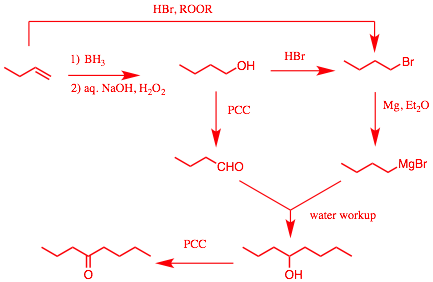
5). Synthesis: (20 pts.) Design a synthesis of the ketone, 4-octanone, using 1-butene as your only source of carbon (4 + 4 = 8 !!!!!). All inorganic or carbon-bearing reagents are available to you. [You will most likely need some reactions from previous chapters.]
Periodic Table
Work Sheet
Work Sheet
Work Sheet