EXAM 1
CHEMISTRY 220
Friday, February 15, 2013
NAME (print): ________________________________________________________
TA:_________________ Sect. Day:_________________ Sect. Time:_____________
Fill in the information above. Place your name on the top right of subsequent pages.
Take a few moments to look over the exam. Answer each question on the exam paper.
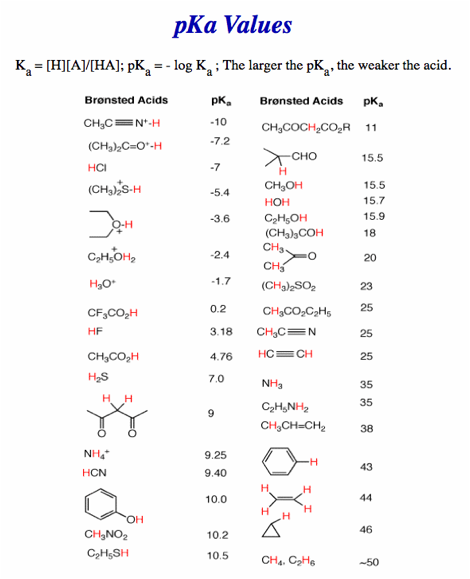
No calculators, electronic devices, earbud texts or notes. You may use molecular models. Important clues and structures are in bold. There is a Periodic Table on pg. 9, a pKa Table on pg. 10 and a BDE Table on pg. 11. Do all preliminary drawing or computations on the work sheets at the end of the exam. The work sheets will not be graded. You may detach the work sheets from the exam.
The exam is 55 minutes.
STOP writing and hand in your exam when you are asked to do so.
Remember: Neatness is to your advantage.
1. (20 pts) Conformation I ______
2. (20 pts) Potpourri (4 of 5) ______
3. (20 pts) Radical Reactions ______
4. (20 pts) Conformation II ______
5. (20 pts) Thermodynamics (3 of 4) ______
______________________________________________
Total (100 pts)
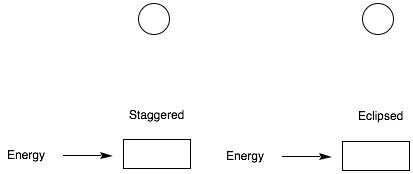
1. Conformation I: (20 pts) For the eclipsed and staggered conformations of 2,3-dimethylbutane viewed along the C2-C3 sigma bond, draw a Newman projection of the least stable eclipsed and staggered conformations. Place the energies for each interaction in the Newman projections below. [Use the circles as templates for the Newman projections.] Calculate the total energy (kcal/mol) of both conformations. Place your answer in the appropriate box. [H/H, eclipsed, 1.0 kcal/mol; CH3/H eclipsed, 1.3 kcal/mol; CH3/CH3, eclipsed, 3.0 kcal/mol; CH3/CH3, gauche, 0.9 kcal/mol] Show your work!
2. Potpourri: (20 pts.; equal weight) Answer 4
of 5 of the following questions. If
you do all five questions, cross out the one that you do not want graded.
a) Estimate the heat
of combustion of n-heptane given that n-butane has a heat of combustion of -687
kcal/mol. Show work. (Hint: What is the heat of combustion of
an unstrained methylene group?)
b) Circle the compounds that are readily deprotonated by HCCNa (sodium acetylide, triple bond between carbons).
methanol CH3CO2H ethylene NH4+ ammonia
c) Circle the species with sp2 hybridized atoms. Use arrows to designate which atoms apply.
HCN
CH2=O
CO2
CH2=C=NCH3
[BeCl3]-1
Continued…..
d) Briefly explain and illustrate why BrHC=C=CHBr has a net dipole moment while
trans - BrCH=CHBr does not.
e) In the series ethane, ethylene and acetylene bond dissociation energy increases while pKa decreases. Explain and/or illustrate.
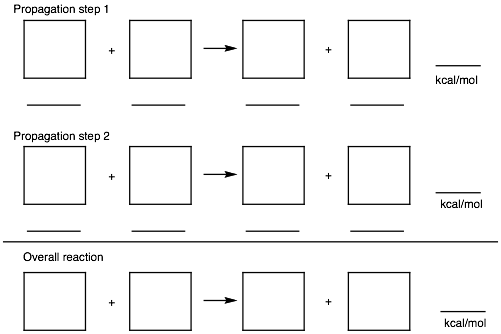
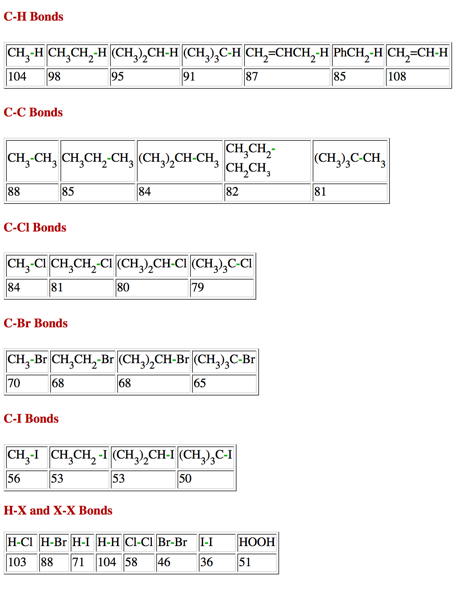
3. Radical Reactions: (20 pts.) Provide the two propagation steps for the free radical monochlorination of 2,2-dimethylpropane (neopentane) using the bond dissociation energies in the BDE Table (pg. 11). Place the reactants and products in the appropriate boxes and the BDEs on the lines. Calculate the heat of each propagation step and the heat of the overall reaction.
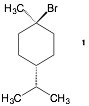
4. Conformation II: (20 pts) Consider the stereoisomer of 1-bromo-1-methyl-4-isopropylcyclohexane
(1) shown here.
a) (10 pts) Draw the chair conformations below so that the
equilibrium arrows are correct. Be
sure all groups are clearly labeled
axial or equatorial.
![]()
chair A
chair B
b) (10 pts) What is the value of D(DGo) for
the above equilibrium given the following A- values for the monosubstituted (X)
cyclohexane: X = Br = 0.6 kcal/mol; X
= CH3 = 1.8 kcal/mol;
X = iso-C3H7 = 2.1 kcal/mol? Show
calculations.
Chair
A energy = ___________
Chair B energy = ___________
D(DGo) = _________________
5. Thermodynamics:
(20 pts.; equal weight) Answer 3 of 4 of the following questions. If you do all four questions, cross out the
one that you do not want graded.
a) The heat of combustion of cis-1,3-dimethylcyclohexane is -1245.7 kcal/mol. The trans stereoisomer has a value of -1247.4 kcal/mol. i) What is the difference in their heats of formation? ii) Which one is more stable? iii) Explain or illustrate the source of the difference in the heats of combustion. Show work.
b) Explain and illustrate the Hammond Postulate.
…..
Continued…
c) For the alkane R-H, illustrate the relationship between bond dissociation energy and the heats of formation of R-H, the R radical and the hydrogen atom.
d) The heat of formation of 3-methylhexane is -46.0 kcal/mol. Estimate the heat of formation of 3-methyloctane. Show work.
Periodic Table
Bond Dissociation Energies (kcal/mol)
Work Sheet
Work Sheet
Work Sheets