_____________________________________________________________________________
Total (222 pts)
Note: The final exam is worth 160 points toward the final grade. The grade shown above will be multiplied by 0.72 to obtain the
adjusted score (222 x 0.72 = 160).
1. Structure : (25 pts.) Compound A (C7H12) reacts with HBr/ROOR (Kharasch Reaction)
to give stereoisomeric compounds B
and C. When compound B is treated with aqueous KOH, compound D (C7H12)
is formed. Under the same conditions compound C forms both A (major)
and D (minor). Ozonolysis and
dimethyl sulfide reduction of D
gives E. Reduction of E with NaBH4 gives
2-methylhexan-1,6-diol. Alternatively,
treatment of A with cat. OsO4/HIO4
produces F (C7H12O2). Reduction of F with LiAlH4 affords heptan-1,6-diol. What are the structures of A-F? Pay attention to
stereochemistry. Provide brief mechanistic
explanations for the formation of A
and D from C and D from B.
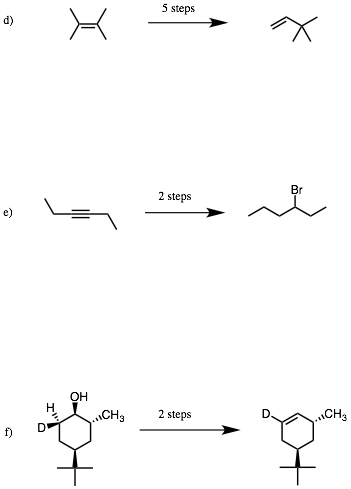
2. Reactions I: (4 x 8 pts. = 32 pts.) Do 4 of 6. Provide the reagents that effect the following transformations. I have listed the number of steps that I would use. Your count may be different and valid. If you do more than four problems, cross out (with a large X through the solution) the ones that you do not want graded.
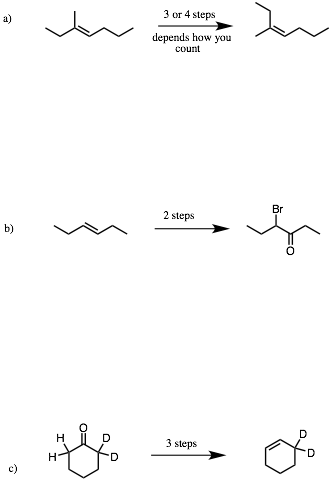
…continued
3. Potpourri: (8 x 5 pts. = 40 pts.) Do 8 of 10 problems. If you do more than eight problems, cross
out (with a large X through the solution) the one(s) that you do not want
graded.
i) Circle the greatest number of compounds that are at the same oxidation level.
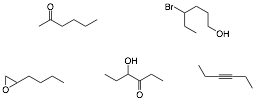
ii) Explain briefly an example how anti-bonding orbitals play a role in organic chemistry.
iii) Ibogamine, of the film Good Will Hunting fame, has the formula C19H24N2. How many rings and double bonds are present? Show work.
iv) The specific rotation of (S,S)-tartaric acid is [a] = -12o. What is the percentage of each enantiomer in a mixture of the two enantiomers that has [a] = +3o. Show work.
…continued
v) The ratio of alkenes formed by
the E2 elimination of 2-bromo-2-methylbutane need not be the same as the ratio
formed when the bromide undergoes E1 elimination during solvolysis. Explain.
vi) Provide a diagram that relates bond dissociation energy and heat of formation for a generic alkane, R-H.
vii) In the free radical chlorination of (S)-1-chloro-2-methylbutane to form dichloropentanes, draw the dichloride(s) that is (are) no longer optically active. Give a ONE WORD explanation why it (they) is (are) no longer optically active.
…continued
viii) Circle the “acids” that are deprotonated by 1-lithiobutyne.
NH3 NH4Cl CH3OH H2O CH4S
ix) Provide an example wherein deuterium labeling provides mechanistic information in organic chemistry.
x) What do the figure 8 knot and the anti conformation of meso-HO2CCH(OH)CH(OH)CO2H have in common? Explain and illustrate.
|
|
4. Synthesis: (25 pts.) We have discussed the pinacol rearrangement in class in the context of 1,2-diols 1 and 2. Design a synthesis of 1 or 2 from cyclohexanone and methyl bromide. All other reagents are available to you. No mechanisms are required. A few concepts that may apply are: C-C bond formation; stereoselective formation of 1,2-diols; hydroboration and oxidation.
5. Thermochemistry: (25 pts.)
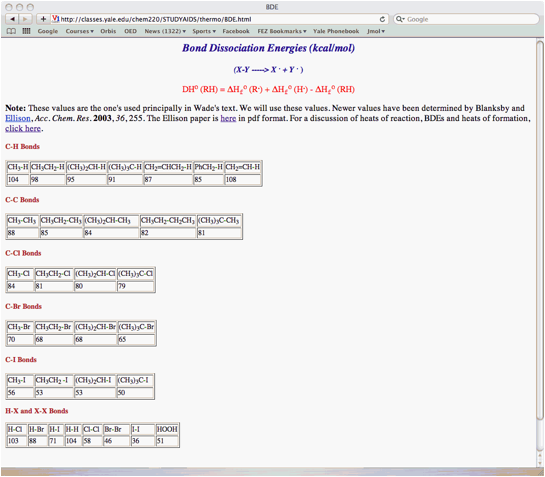
a) (15 pts.) Determine the heat of formation of chlorocyclohexane prepared by the free radical chlorination of cyclohexane. Provide the two propagation steps and the overall reaction for this process. Place the reactants and products in the appropriate boxes. Compute the heats of each reaction using the data in the BDE Table (pg. 17).
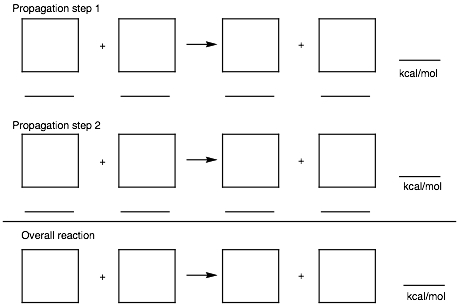
b) (10 pts.) Calculate the heat of formation of chlorocyclohexane using the following data: DHfo (HCl) = -22.1 kcal/mol; DHfo (cyclohexane) = -29.5 kcal/mol. Show work.
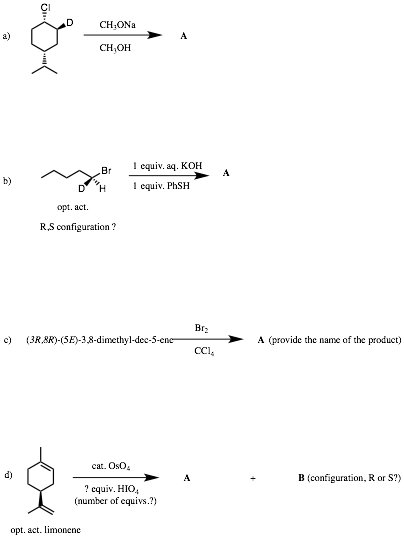
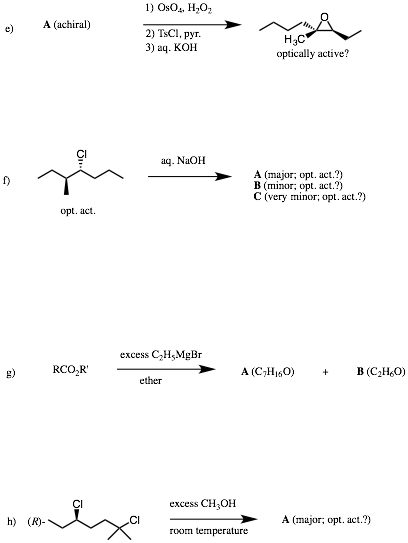
6. Reactions II: (30 pts.) Do 6 of 8. Provide structures for the unknown compounds AND answer the ? marks. Be explicit about stereochemistry, optical activity, racemates, etc. No mechanisms required! If you do more than 6 problems, cross out (with a large X through the solution) the one(s) that you do not want graded.
…continued
7. Kinetics/Thermodynamics: (20 pts.) Do one from pg. 12 and one from pg. 13. Keep your answer short and to the point! If you do more than two problems, cross out (with a large X through the solution) the one(s) that you do not want graded.
a) “The more stable conformation of a compound is always the one that reacts faster because there is more of it.” True or False? Provide an example.
b) Explain the difference
between a kinetically-controlled and
thermodynamically-controlled reaction. Be
brief. A diagram will help.
…continued
c) In the SN1 solvolysis reaction of tert-butyl bromide the rate is expressed as k[RBr]. The solvent does not enter the rate expression. Explain and illustrate briefly.
d) What is the Hammond Postulate? Briefly explain and illustrate.
8. Mechanism: (25 pts; equal weight) In PS9, exam 3 and in class (4/15 and 4/29) we considered the Stevens oxidation using aqueous NaOCl in acetic acid (HOCl). The authors observed that 2o-alcohols are oxidized much faster than 1o-alcohols. In a subsequent paper the authors demonstrated this selectivity by converting diol 1 into keto alcohol 2. I suspect that they prepared diol 1 from the enantiomer of the monoterpene a-pinene (3).
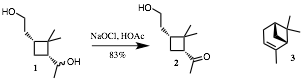
a) How would you convert 3 to 1?
b) Give the two R, S-designations in diol 1. (Ignore the secondary alcohol carbon since 1 is a mixture of optically active diastereomers.) Put your answer by structure 1.
c) In the Stevens’ paper of PS9 a table showed that the primary alcohol, n-decyl alcohol (1-decanol), was efficiently converted into the ester, n-decyl decanoate (C20H40O2) in the presence of aq. NaOCl/HOAc. Provide a mechanism for this reaction. [Use R = C9H19; RCH2OH = n-decanol] [ORGO, alcohols, #3, exam 3; also discussed in class on 4/15 and 4/17.]
d) In the second paper the authors demonstrated that methanolic NaOCl/HOAc was capable of oxidizing heptanal (n-heptyl aldehyde) to methyl heptanoate (n-C6H13CO2CH3). The success of this reaction tells you what about the rate of oxidation of methanol relative to primary alcohols and secondary alcohols using this oxidant? Be brief.
e) Do you think that the oxidation in d) will work successfully in the conversion of oleic aldehyde [(Z)-octadec-9-enal] to methyl oleate? Explain and illustrate.
Periodic Table
Work Sheets
Work Sheets