EXAM 3
CHEMISTRY 225b
Friday, April 25, 2008
NAME (print): ___________________________________________________
TA:_____________________ Day:_________________ Time:__________
Take a few moments to look over the exam. Answer each question on the exam paper.
Important clues, points, and structures are in bold.
Do all preliminary drawing or computations on the work sheets at the end of the exam. The work sheets will not be graded
The exam is 55 minutes.
STOP writing and hand in your exam when you are asked to do so.
Remember: Neatness is to your advantage.
1. (30 pts) Reactions ______
2. (30 pts) Potpourri ______
3. (20 pts) Synthesis ______
4. (20 pts) Structure ______
_____________________________________
Total (100 pts)
1. (30 pts) Reactions: Provide answers to the following reactions.
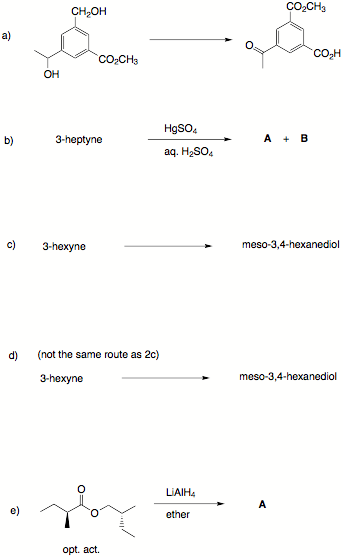
2. (30 pts.) Potpourri:
Complete each of the following questions.
a) Stable solutions of the
lithium salt of 1-octyne can be prepared in which of the following solvents (Circle your answer(s)).
(E)-3-hexene
H2O
acetone
NH3
(CH3)3COH
b) Lindlar reduction of
2-pentyne liberates 37.4 kcal/mol of heat. Hydrogenation of (E)-2-pentene liberates 27.4 kcal/mol of heat to form n-pentane (DHfo=-35.1
kcal/mol). Circle your best estimate for the heat of formation of 2-pentyne
(kcal/mol)? (What do you
know about the DHfo
of (E)- and (Z)-disubstituted alkenes?)
-29.7 -30.7 +29.7 +30.7 -7.7
c) Circle the halide(s) that will form viable Grignard
reagents.
BrCH2CH2Br HOCH2CH2CH2Br
CH3CH2OCH2CH2CH2Cl
BrCH2CH2CHO BrCH2CH2CO2H
d) Circle the greatest number of compounds that are at the same oxidation level.
![]()
e) Circle the products expected to be formed during the hydrolysis of methyl benzoate with H318O+.
![]()
3. (20 pts) Synthesis: Design a synthesis of (±)-epoxide 1 using 2-butyne as your only source of carbon. All other reagents are available to
you. [Hint: You did most of this problem on your
homework.]
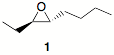
4. (20 pts). Structure:
Optically-active alkyne A,
C10H18, undergoes reduction to form compound B (C10H20). Compounds A or B
form a single (S)-carboxylic
acid (C5H10O2) C upon oxidation with aqueous KMnO4. [At this point you should know the
structures of A and C and some of B]. Compound B also
reacts with bromine to form two
(and not one) optically active dibromides D and E
(D and E are not distinguishable). What are the structures of A-E? Show
how D and E determine the stereochemical issue in B. How
was A reduced to B?
Work Sheets
Work Sheets
Work Sheets