EXAM 2
CHEMISTRY 225b
Friday, March 28, 2008
NAME (print): ___________________________________________________
TA:_____________________ Day:_________________ Time:__________
Take a few moments to look over the exam. Answer each question on the exam paper.
Important clues, points, and structures are in bold.
Do all preliminary drawing or computations on the work sheets at the end of the exam. The work sheets will not be graded
The exam is 55 minutes.
STOP writing and hand in your exam when you are asked to do so.
Remember: Neatness is to your advantage.
1. (30 pts) Reactions (5 of 6) ______
2. (30 pts) Potpourri ______
3. (20 pts) Structure ______
4. (20 pts) Mechanisms (1 of 2) ______
_____________________________________
Total (100 pts)
1. (30 pts.) Reactions:
Provide the required information in 5 of 6 of the following reactions. Pay attention to stereochemistry, optical activity, etc. If you do six problems, cross
out the one that you do not want graded.

2. ContinuedÉ

2. (30 pts.) Potpourri: Complete each of the following problems.
a) (R, R)-Tartaric acid has [a] = +12o. What is the specific rotation of a 2:1 mixture by weight of meso-tartaric acid and (S, S)-tartaric acid, respectively? Show work.
b) Circle the compounds that are capable of resolution.
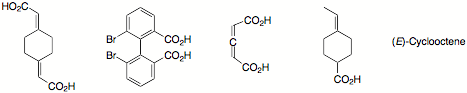
c) Circle the terms that apply to E2 eliminations.
concerted deuterium isotope rate=k[RX]
effect
orbital overlap rate=k[RX][Nuc]
d) Circle the alcohol(s) that represent the racemates formed by the hydroboration of (E)-3-methyl-2-hexene.

e) The normal chain hexene that gives a single product upon ozonolysis and is more stable by ~1 kcal/mol than its geometrical stereoisomer.
3. (20 pts.) Structure: Compound A, C8H16, forms a meso compound B upon reaction with bromine in CCl4 and forms d,l-diol C upon oxidation with OsO4/H2O2. Ozonolysis of A forms a single ketone D. What are the structures of A-D? Show your reasoning.
4.
(20 pts.) Mechanisms:
Provide a detailed mechanism for one, and only one, of
the reactions shown below. Use the curved arrow formalism. Pay attention to stereochemistry, and the absence of stereo- and
regioisomers as these issues may apply.

Work Sheets
Work Sheets
Work Sheets