EXAM 4
Comprehensive Organic Chemistry
CHEMISTRY 225b
Friday, April 21, 2006
NAME (print): _______________________________________________________________
TA:_____________________ Section Day:_________________ Section Time:__________
No Calculators! Take a few moments to look over the exam. Answer each question on the exam paper. Chemical Shift Tables are on pages 8 and 9.
Important clues and structures are in bold.
Do all preliminary drawing or computations on the work sheets at the end of the exam. The work sheets will not be graded.
The exam is 55 minutes.
STOP writing and hand in your exam when you are asked to do so.
Remember: Neatness is to your advantage.
1. (25 pts) Structure/NMR Spectroscopy ______
2. (25 pts) Potpourri (4 of 5) ______
3. (25 pts) Reactions (4 of 5) ______
4. (25 pts) Synthesis/Mechanism: ______
___________________________________________
Total (100 pts)
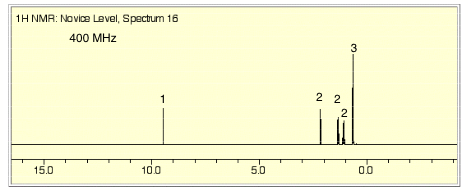
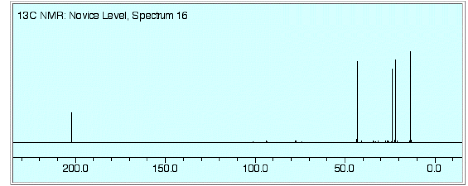
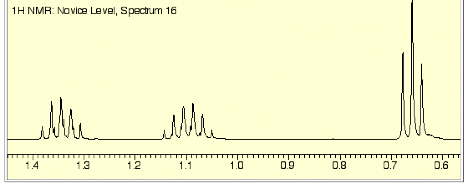
1. (25 pts) Structure/NMR Spectroscopy: The NMR spectra for an unknown compound A (C5H10O) are provided on page 3. [The numbers above the signals in the 1H NMR spectrum are the relative areas. Blow-ups of the 1H NMR spectrum, which are not scaled to one another, are on page 4. Ignore baseline “blips” in the 13C NMR spectrum.]
a) (7 pts) Deduce the structure of A.
Show your reasoning. [Helpful
hints: Degree of unsaturation? Unique
carbons? Chemical shifts of some signals will tell you what functional groups
are present? What atoms are left once the functional group has been
established? Coupling patterns? This problem is from PS9 and the NMR module.]
b) (6 pts) Assign the five signals in the 1H NMR to
the hydrogens in A. Redraw the structure of A on
the 1NMR spectrum on page 3 and connect arrows from the hydrogens to
the appropriate signals.
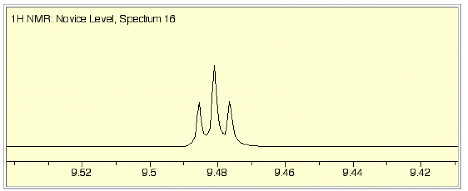
c) (6 pts) The 1H NMR
spectrum has two different coupling constants as shown in the middle blow-up
(page 4). Provide the chemical
shift and multiplicity (s, d, t, tq, etc?) of this resonance. Put your answer on the spectrum on
page 4.
d) (3 pts) The larger of the
coupling constants in c) is ~8 Hz.
Show how this value can be determined from the d scale hash-marks and the frequency of the spectrometer. Answer on page 4.
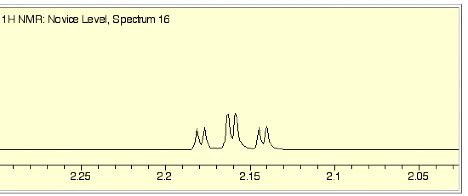
e) (3 pts) Estimate the
smaller coupling constant in d). Answer
on page 4.
These blow-ups are not to scale, one to another. Each one is internally consistent to its d-scale.
2.
(25 pts) Potpourri: Answer 4 of
5 of the following questions. If you answer all five, cross out
the one that you do not want graded.
a) Circle the compounds that will have an odd number of singlets in their
broadband decoupled 13C NMR spectrum.
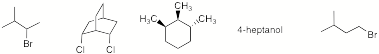
b) The 1H NMR spectrum of ethyl iodide recorded at 90 MHz displays J = 7.0 Hz. What is the value of J at 180 MHz?
c) Circle the major product from the oxidation of 1-butanol with PCC in aq. CH2Cl2.
![]()
d) A single hydrogen in a 1H NMR (100 MHz) spectrum displays four peaks as shown (d values). What are the chemical shift, multiplicity (s, d, t, td, etc?) and J values for this signal?
e) In what way, other than chemical shift,
are the broadband decoupled 13C spectra of tribromides 1 and 2
distinguishable? Explain briefly.
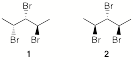
3. (25 pts) Reactions: Complete four of
the following questions. If you do
all five, cross out the one you
do not want graded. Pay
attention to stereochemistry, optical
activity, etc.

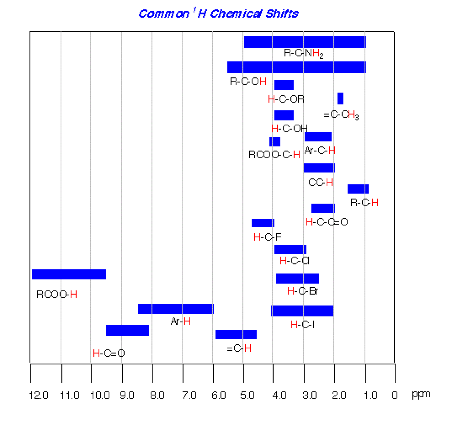
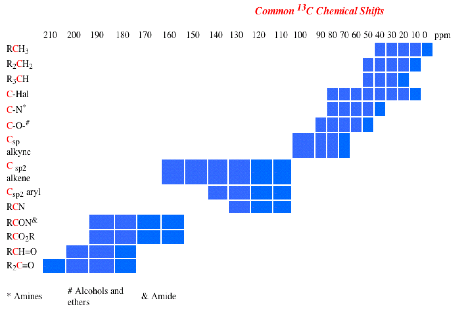
4. (25 pts) Synthesis/Mechanism: Two Chem 225b students have positions in a laboratory during the summer break. They are charged with preparing chiral ether 1 from (R)-2 and cyclohexanone (3) as the only sources of carbon in 1. They both reject acid-catalyzed reactions and settle on two alternative approaches using the Williamson ether synthesis. His fails; hers succeeds. What did she do to be successful? [All reagents are available.]
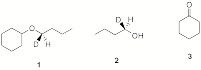
Work Sheets
Work Sheets
Work Sheets