FINAL EXAMINATION
Comprehensive Organic Chemistry
Chemistry 225b; 2 P.M., Thursday, May 4, 2006
OML 202
NAME (print): ______________________________________________________________
TA:_____________________ Section Day:_____________ Section Time:______________
Take a few moments to look over the exam. Do problems first with which you are most comfortable. Important points and unknowns are in bold type. Do all preliminary work on the worksheets. The worksheets will not be graded. The exam is the length of two hour exams with an additional one hour for review. STOP writing when you are asked to do so. Put your name at the top of the cover sheet and subsequent pages (except for Work Sheets).
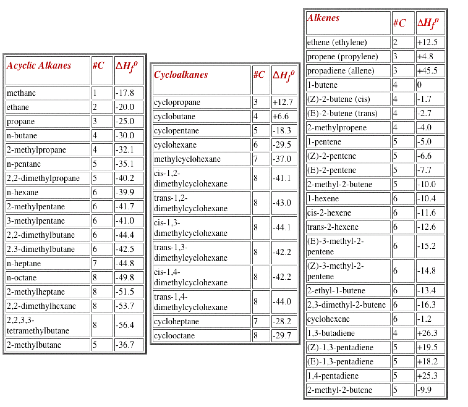
There is a Periodic Table on page 14. Tables of Heats of Formation are on page 15.
Remember: Neatness is to your advantage.
Have a GREAT summer
and do well in the second term of ORGO!
1. Structure/Spectroscopy ________ 5. Mechanism/Stereochemistry
(26 pts) /Oxidation (36 pts) _________
2. Structure (26 pts) _________ 6. Elimination Reactions (30 pts) _________
3. Reactions (6 of 8) _________ 7. FAQs (30 pts) _________
(36 pts)
4. Potpourri (6 of 8) _________ 8. Thermochemistry (30 pts) _________ (36 pts)
_______________________________________________________________________
Total (250 pts)
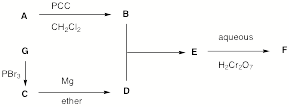
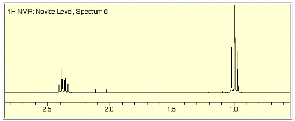
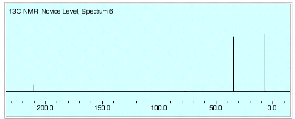
1. Structure/Spectroscopy: (26 pts) Compounda A and G undergoes the series of reactions shown in the scheme below. The NMR spectra of F are provided. What are the structures of A-G? Explain and illustrate your reasoning. [Ignore the “blips” in the 1H NMR spectrum between d 2.0 and 2.2.]
2. Structure: (26 pts) Hydrocarbon (S)-A (MW = 84) [Hint: How many carbons would be in A if there were no hydrogens?] reacts with hydrogen in the presence of a catalyst to liberate ~30 kcal/mol (page 15?) of heat forming achiral alkane B (4 singlets in its broadband decoupled 13C spectrum). Exposure of compound A to HBr provides C, D and E. Achiral compound E is a constitutional isomer of C and D, which are optically active stereoisomers of one another. Free radical bromination of B provides E as the major (>99%) product. When C or E is exposed to EtONa/EtOH, achiral (E)-F is formed as the major product. Under the same conditions, D affords achiral (Z)-F. Compound (E)-F liberates less heat on hydrogenation in forming B than does (Z)-F. What are the structures A-F? Explain and illustrate.
3. Reactions: (6 x 6 = 36 pts) Do 6 of 8. If you do all of them, cross out the ones you do not want graded.
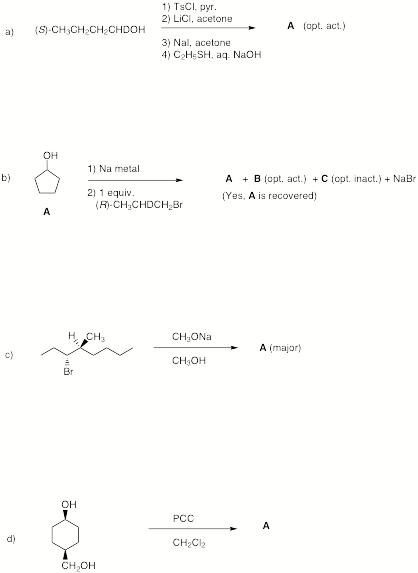
3. Continued…
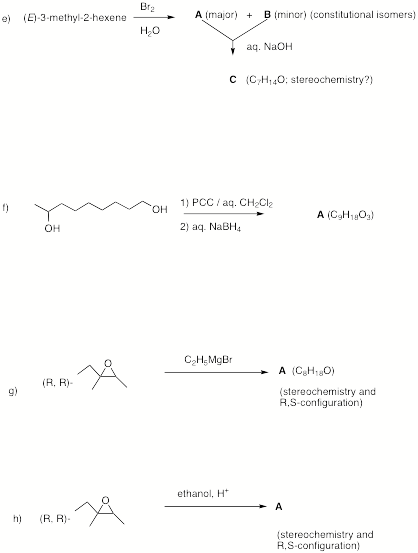
4. Potpourri: (6 x 6 = 36 pts) Do 6 of 8. If
you do all of them, cross out the
one you do not want graded.
a) Number the following carbonyl groups in order of decreasing infrared energy (wave number, cm-1). [1 = highest; 5 = lowest]
acyl halide ester aldehyde ketone amide
b) Consider the free radical monochlorination products of 2,3-dimethylbutane. How much of each is formed (a ratio is fine), and which ones are achiral, optically active or racemic? [relative reactivity: 3o = 5; 2o = 4; 1o = 1. ] Show work.
c) Estimate the heat of formation of (E)-2-octene [page 15?]. Show work.
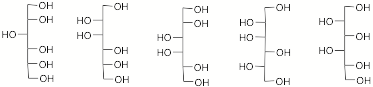
d) How many singlets will appear in the broadband decoupled 13C NMR spectrum of each of the hexitols shown below in Fischer projections? Place the number under the structure AND circle the two that are identical.
4. Continued …
e) Rank the following types of spectroscopy from the user
of the highest electromagnetic energy (5) to the lowest electromagnetic energy
(1).
IR 1H
NMR UV visible 13C
NMR
f) A solution of “natural” (R,R)-tartaric acid has [a]D
= +12o. What is the
value of [a]D when the concentration
of the sample is halved?

g) The total width of a triplet in a 1H NMR spectrum
recorded at 500 MHz is 0.01 d-units. What is the value of J in Hz? Illustrate and show work.
h) Number the following
compounds in order of increasing acidity. [Least (1); Most (5)]
NH3 HOAc ethanethiol 1-butyne isopropanol
5. Mechanism/Stereochemistry/Oxidation: (6 x 6 pts = 36 pts) We have discussed the oxidation of primary alcohols with PCC and CrO3.2pyr. in anhydrous CH2Cl2 as effective methods for forming aldehydes but not without possible side reactions (Exam 4, 3b). When 1,4-butanediol (2) is oxidized with PCC in anhydrous CH2Cl2, the dialdehyde 1 is not the major product of the reaction, but rather the intramolecular ester, g-butyrolactone (3).

a) Knowing what you know about the interaction of water (which isn’t present) and its analogs with aldehydes, provide a mechanism that includes a carbonyl-free intermediate in the formation of 3 from 2.
Similarly, optically-active dihydroxyaldehyde 4 reacts with PCC to form the bridged ketolactone 5.
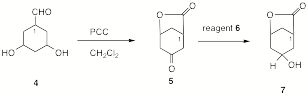
b)
If C1 of ketolactone 5 is of
the (R)-configuration, draw 4 with stereochemistry (bold/dotted lines) in template 4’ and circle 5a or 5b as the correct
enantiomer of 5.
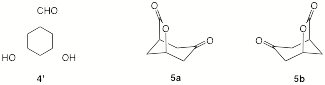
5. Continued…
c) Owing to the steric effect of the bridged -C(O)O- group in the transition state for reduction, exposure of ketolactone 5 to reagent 6 is expected to provide principally optically-active hydroxylactone 7. Place your choice of reagent 6 in the box and circle the correct stereoisomer for 7.
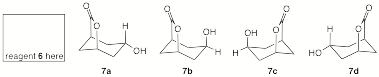
d) The conversion of ketolactone 5 to hydroxylactone 7 can be monitored over time by infrared spectroscopy. One of the two carbonyl absorptions (1775 and 1710 cm-1) in the IR spectrum of 5 will disappear and be replaced by an absorption at ~3300 cm-1 in 7. Assign these three stretching frequency absorptions to their respective bonds.
e) Show how 1H NMR spectroscopy can be used to assign the stereochemistry of the hydroxyl group (through its methine hydrogen) in 7. [180o, J = 12 Hz; 90o, J = 0 Hz; 60o, J = 4 Hz. Assume only these dihedral angles apply and that the –OH does not couple to the methine hydrogen. Show a coupling pattern. [Hint: The total width of the pattern is 16 Hz. Is this in accord with your structure for 7?]
f) Treatment of
optically active 7 with catalytic H2SO4
is expected to give racemic 7. Illustrate this process with a
mechanism.
6. Elimination Reactions: (30
pts) In exam 3, the following
question appeared in Potpourri.
Few students had the courage to circle all five of the choices as
correct answers. Explain and
illustrate how each of these terms apply to E2 reactions. You may use more than one illustration.
Circle the terms that may apply to E2
reactions.
0o dihedral
angle / concerted bond cleavage / isotope effect / bimolecular /
kinetically controlled
7. FAQs: (3 x 10 pts =
30 pts) Here are some questions that I hear frequently from students. You provide a concise explanation. Do
3 of 4. If you do all of them, cross
out the one you do not want graded.
a) Although we can determine that
meso-tartaric acid is optically inactive by passing a mirror plane through one
of its eclipsed conformations, there is little eclipsed conformation present. What gives? [tartaric acid = HOOCCH(OH)CH(OH)COOH]
b) I don’t understand why resolution
[separation of signals] improves in 1H NMR spectra as we go from,
say, 60 MHz to 500 MHz.
7. Continued…
c) How do I know if there is going to be
more SN1 or E1 product?
d) Why does the acidity of C-H bonds
increase [pKa decrease] and BDEs increase in the series ethane, ethylene and
acetylene?
8. Thermochemistry: (30 pts) The Standard State diagram below locates
three hydrocarbons (A, B
and C) in terms of their
relative heats of formation (page 15?).
Identify A, B and C
and name the quantities a-g. There are no calculations required and
there is sufficient information to determine the structures.
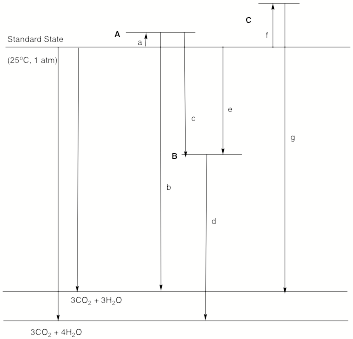
A: c:
B: d:
C: e:
a: f:
b: g:
Periodic Table
Heats of Formation
Work Sheet
Work Sheet
Work Sheet
Work Sheet