NAME (print): ___________________________________________________
TA:_____________________ Day:_________________ Section Time:__________
Important points are in bold.
Complete the section above and put your name on pages 2-5.
Take a few moments to look over the exam. Answer each question on the exam paper.
Do all preliminary drawing or computations on the Work Sheets at the end of the exam.
They will not be graded.
A Periodic Table is on page 6 of the exam should you need it.
The exam is 55 minutes.
STOP writing when you are told to do so.
REMEMBER: Neatness is to your advantage.
1. (28 pts) ______
2. (28 pts) ______
3. (22 pts) ______
4. (22 pts) ______
______________________________
Total (100 pts)
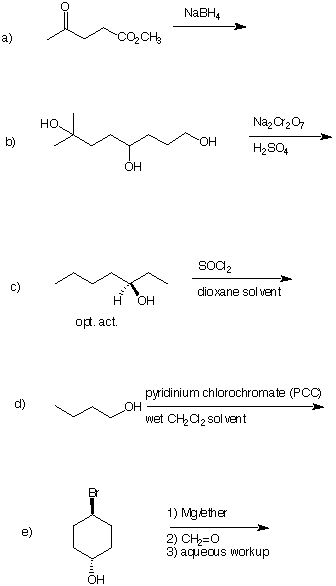
1) (28 pts) Provide the structure of the expected product in four of the following five reactions. If you do five of them, cross out the one you do not want graded. [Otherwise the choice is ours!]

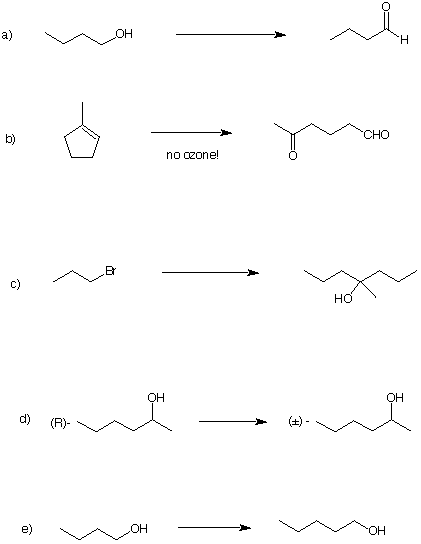
2) (28 pts) Provide conditions for the completion of four of the following five reactions. If you do five of them, cross out the one you do not want graded. [Otherwise the choice is ours!] All reagents and
any other carbon sources you may need are available to you. Place the numbered reagents (as shown in
question 1e) above and below the arrow.
3) (22 pts) In the acid-catalyzed rearrangement of pinacol (2,3-dimethylbutan-2,3-diol), only the ketone pinacolone (3,3-dimethyl-2-butanone) is formed. However, the acid-catalyzed rearrangement of 1,2-diol 1 gives ketone 2 (major product) and a second pinacol rearrangement product, ketone 3.

a) (9 pts) Provide a mechanism (curved arrows) for the formation of 2 from 1.
b) (9 pts) What is the structure of 3? Place it in the box.
c) (4 pts) What alkyl group migrates, i.e., rearranges, in the formation of 3?
|
4) (22 pts) A chemist requires a sample of racemic 3-methyl-3-heptanol (1). Fully aware of the chemistry of Grignard reagents, she designs and executes a synthesis of 1 using 1- butene as the sole source of carbon. Show how she may have accomplished her goal. Naturally, all reagents were available to her as they are to you.
|
|