NAME (print): ___________________________________________________
TA:_____________________ Day:_________________ Section Time:__________
Important points are in bold.
Complete the section above and put your name on pages 2-6.
Take a few moments to look over the exam. Answer each question on the exam paper.
Do all preliminary drawing or computations on the Work Sheets at the end of the exam.
They will not be graded.
A Periodic Table is on page 8 of the exam should you need it.
The exam is 55 minutes.
STOP writing when you are told to do so.
Remember: Neatness is to your advantage.
1. (25 pts) ______
2. (20 pts) ______
3. (25 pts) ______
4. (30 pts) ______
_______________________________
Total (100 pts)
1) (25 pts) Dr. Know-It-All attempts to prepare ethyl iodide by the free radical chain iodination of ethane. After all, he knows that it works for chlorination and bromination. However, he notices that the red color of iodine never disappears and no ethyl iodide is being formed. Using propagation steps, BDEs, heats of reaction for each propagation step and the overall reaction, and an energy diagram that uses these heats and obeys the Hammond Postulate, provide a brief explanation for Dr. Know-It-All's dilemma. BDEs.
(5 pts) propagation step 1:
--->
DHo = _______ kcal/mole
(5 pts) propagation step 2:
--->
DHo = _______ kcal/mole
_____________________________________________________________________________
(5 pts) overall reaction:
--->
DHo = _______ kcal/mole
Problem 1 continued. Energy diagram (5 pts)

Brief Explanation (5 pts):
2) (4 x 5 pts = 20 pts) Allyl bromide 1 is a lachrymator. Exposure of allyl bromide vapors to the moist eye creates tearing and a burning sensation. 1-Bromopropane does not have this property.
![]()
a) Use your knowledge of the SN1 reaction to explain briefly why 1 exhibits this property and 1-bromopropane does not.
b) With the aid of p-orbitals and lines for single bonds, draw the structure of the reactive intermediate formed from 1. Give a reason for its stability.
c) Write a balanced equation for the reaction of 1.
d) What do you think causes the burning sensation?
3) (25 pts) Citronellol 1 appears in Ceylon and Java citronella oil as the enantiomer with [a]D20 = +5.22o. The other enantiomer is present as a constituent in rose and geranium oil with a reported specific rotation of [a]D20 = -4.76o. Assume that the dextrorotatory enantiomer is pure and that it contaminates the levorotatory enantiomer.
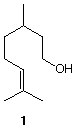
a) (10 pts) How much of each enantiomer is present in the contaminated sample? Show work.
b) (5 pts) What is the optical purity (enantiomeric excess) of the contaminated sample? Show work.
c) (5 pts) Draw the (R)-enantiomer.
d) (5 pts) Is the (R)-enantiomer the dextro- or the levorotatory enantiomer? Explain briefly.
4) (6 x 5 pts = 30 pts) Predict the major product in each of the following reactions. Comment very briefly.
