EXAM 4
CHEMISTRY 220
Friday, December 5, 1997
NAME (print): ___________________________________________________
TA:_____________________ Day:_________________ Time:__________
Important points are in bold.
Take a few moments to look over the exam. Do the questions first with which you feel most comfortable.
Answer each question on the exam paper.
Do all preliminary drawing or computations on the work sheets at the end of the exam.
There is a Periodic Table of the Elements at the end of the exam.
The exam is 55 minutes.
STOP writing when you are asked to do so.
REMEMBER: Neatness is to your advantage.
1. (20 pts) ______
2. (20 pts) ______
3. (20 pts) ______
4. (20 pts) ______
5. (20 pts) ______
________________________________
Total (100 pts)
1. (4 x 5 = 20 pts) Draw the structure of the major product in each of the following
reactions. Pay very careful attention to stereochemistry where it is required. If
there is no obvious stereochemical control in the reaction, make this information
known. Comment briefly.

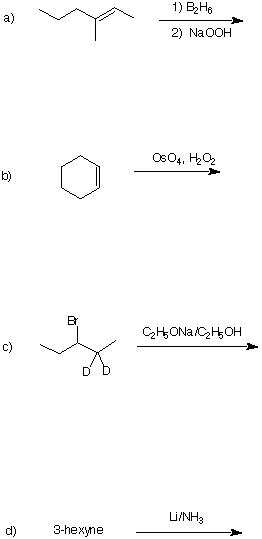
2. (20 pts) Design a synthesis of racemic (not meso) oxirane 1 using acetylene and
ethanol as the only source of carbon in 1. All reagents are available.
3. (4 x 5 = 20 pts) Compound A (C6H12) affords compounds B and C upon
ozonolysis. The 1H NMR spectrum of B is shown below. Relative areas are indicated
in circles on the spectrum. [d 1.05 (3H, triplet, J = 7 Hz), 2.15 (3H, s) and 2.45 (2H, quartet, J = 7 Hz)]
a) What is the structure of B?
b) What is the structure of C? Explain briefly.
c) What structures are possible for A? Explain briefly.
d) The spectrum of B was recorded at 60 MHz (J = 7 Hz). What will be the value of
J at 180 Mz? Explain briefly.
4. (20 pts) When (R)-1-chloro-2-methylbutane undergoes free radical chlorination, two
of the dichlorides that are formed are optically inactive. The proton decoupled 13C
NMR spectrum of dichloride A shows four singlets and dichloride B displays five
singlets. What are the structures of A and B? Explain.
5. (4 x 5 = 20 pts) Circle the best answer in each of the following:
a) The proton with the smallest d value
RCHO R2C=CRH RCH2R RCH2OR
b) The compound with the most singlets in its decoupled 13C NMR spectrum
c) The weakest base
NH3 RCN R3N H2O
d) The compound at the same oxidation level as propyne
![]()