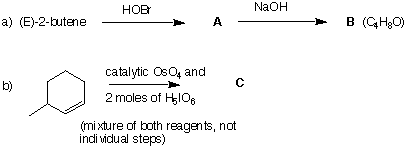
EXAM 4
CHEMISTRY 220
Friday, December 6, 1996
NAME (print): ___________________________________________________
TA:_____________________ Day:_________________ Time:__________
NOTICE: The make-up exam for those individuals with a valid dean's excuse will be held on Wednesday, December 11 at 9:30 A.M. (not 9: 00 A.M. as indicated in the syllabus) in 110 SCL. The final exam is scheduled for Friday, December 20, at 2 P.M. Report to your usual exam room: A-Lee, 110 SCL; Leung-Tesfaye, 59 SPL; Thomas - Z, 18 SCL.
Take a few moments to look over the exam. Important points and unknowns are in bold type. Answer each question on the exam paper. Do all preliminary work on the three blank sheets at
the end of the exam. This section of the exam will not be graded. The exam is 55 minutes. The points should be an indication of the time required for solution. STOP writing when you are asked to do so. REMEMBER: Neatness is to your advantage.
1. (20 pts) ______
2. (24 pts) ______
3. (20 pts) ______
4. (16 pts) ______
5. (20 pts) ______
--------------------------------------------------------------------
TOTAL (100 pts) ______
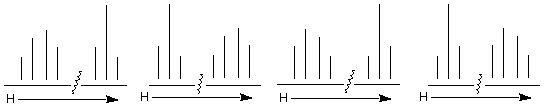
1. (20 pts) An alkyl bromide A, C4H9Br, has the 1H NMR spectrum shown below. Blow-
ups (not to scale) of each signal appear at the top of the spectrum. The signal at d 1.0 accounts for six hydrogens.
a) (15 pts) Draw the structure of A and assign chemical shifts to each of the hydrogen
atoms.
b) ( 5 pts) Why is the signal at d 2.0 a nonet?
2. (6 x 4 pts = 24 pts) Provide brief answers to the following questions. A few well-chosen
words will suffice.
a) What is the effect on J, the coupling constant, when a 1H NMR spectrum is recorded at
60 MHz vs. 500 MHz?
b) What is the range of chemical shifts (ppm) in 1H vs.13C NMR?
c) How many signals does 1-bromo-2-ethylbutane display in its decoupled 13C NMR
spectrum? Why?
d) How does nicotinamide adenine diphosphatide (NAD+) distinguish between enantiotopic hydrogens in ethanol?
e) What evidence exists that nature's methylating agent, S-adenosyl methionine (SAM), alkylates by an SN2 mechanism?
f) In the 60 MHz 1H NMR spectrum of acetone, the protons appear as a singlet at d 2.2. What is the d value when the spectrum of acetone is recorded at 500 MHz?
3. (4 x 5 pts) Provide the principal product (A-F) in each of the following reactions. Pay
attention to stereochemistry, etc. Comment very briefly on the essential point(s) of each reaction.
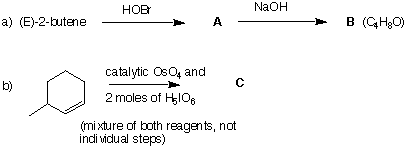
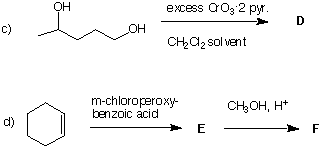
4. (16 pts) A classmate proposes the synthesis of (R)-citronellal (4) from (S)-6-methyl-5-
hepten-2-ol (1). You point out that the first transformation will work but the two
subsequent transformations are conceptually flawed. Provide a brief critique of the three transformations. [p-TsCl = p-toluenesulfonyl chloride. It is understood that the reaction (R)-2 ---> (R)-3 involves treatment with water before isolation of 3. A "transformation" proceeds from one structure to another.]
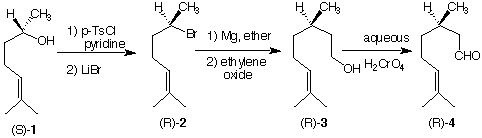
Critique (S)-1 --> (R)-2:
Critique (R)-2 --> (R)-3:
Critique (R)-3 --> (R)-4:
5. (5 x 4 pts = 20 pts) Circle the best answer for each of the following statements.
a). The type of proton with the largest value of d.
CH3OR RCH2Cl Ph-H CH2=CH2
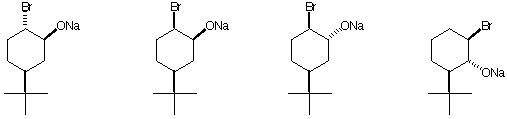
b) The bromo alkoxide that forms an epoxide the fastest.
c) The substance with the fewest number of signals in its decoupled 13C NMR spectrum.
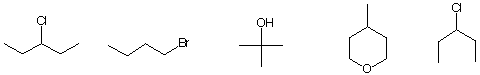
d) The most highly oxidized compound.

e) The 1H NMR coupling pattern most representative of an isolated ethyl group.