EXAM 3
CHEMISTRY 220
Friday, December 2, 2011
Answer Key
NAME (print): ________________________________________________________
TA:_________________ Sect. Day:_________________ Sect. Time:_____________
Fill in the information above. Place your name on the top right of subsequent pages.
Take a few moments to look over the exam. Record answers to questions on the exam paper.
No calculators, electronic devices or earbuds. You may use molecular models. Important clues and structures are in bold. A Periodic Table (pg. 8) is provided.
Do all preliminary drawing or computations on the work sheets at the end of the exam. The work sheets will not be graded. You may detach the work sheets from the exam.
The exam is 55 minutes.
STOP writing and hand in your exam when you are asked to do so.
Remember: Neatness is to your advantage.
1. (10 pts) Mechanism ______
2. (30 pts) Potpourri (do 3 of 5) ______
3. (40 pts) Potpourri (do 4 of 6) ______
4. (20 pts) Synthesis ______
______________________________________________
Total (100 pts)
1. Mechanism: (10 pts.) From the reading assignment on PS9, write a mechanism for the following reaction.
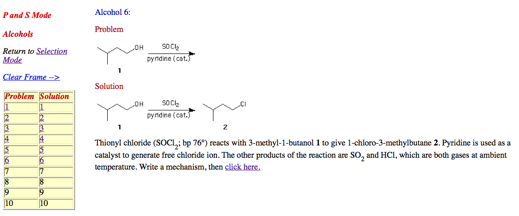
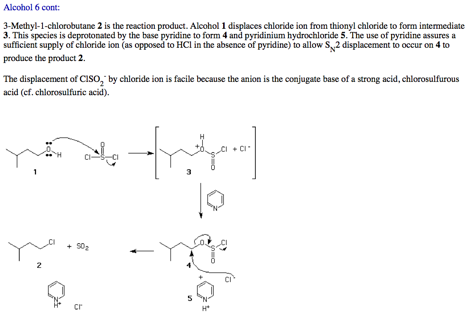
2. Potpourri:
(30 pts., equal weight) Complete 3 of 5 of the following questions. You may do 3c or 3d, but not both of them. If you do more than three
questions, cross out the ones that you do not want graded.
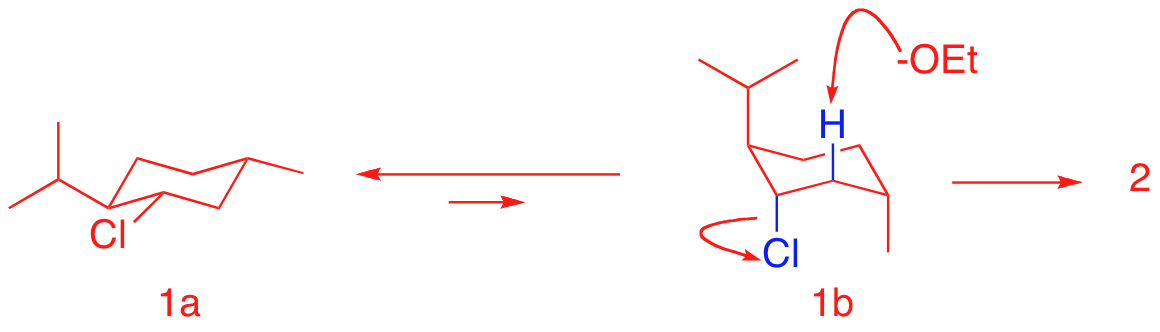
a) Explain why the small, unhindered base sodium ethoxide does not give any of the more substituted alkene (Zaitsev product). A knowledge of chair conformations and the E2 mechanism is useful.
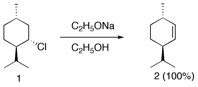
Conformation 1a has all equatorial groups.
There is no hydrogen having a 180 degree dihedral angle between H and
Cl. The less stable conformation
1b has one, giving the less substituted double bond of alkene 2.
b) The oxidation of 2,3-dimethyl-2-butene to acetone with OsO4/H5IO6 is a four-electron oxidation. The same reaction may be conducted with ozone followed by dimethyl sulfide reduction. Explain and illustrate the number of electrons involved in the O3/(CH3)2S reaction.
Both procedures are 4-electron oxidations. Since dimethyl sulfide is converted to
dimethyl sulfoxide (a 2-electron loss), the ozonide must be reduced (2-electron
gain). Therefore, the formation of
the ozonide from the alkene must be a 6-electron loss (oxidation). Thus, -6 + 2 = -4 electrons.
Écontinued
c) Circle the terms that are applicable to an E2 reaction.
concerted deuterium isotope effect intermediate
rate =k[RX][base] preferred syn elimination reversible reaction
d) Circle the alkene reactions that are stereospecific.
OsO4/NMMO HBr/cat. R2O2 peracid oxidation
acid-catalyzed hydration hydroboration
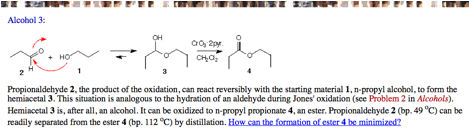
e) Oxidation of 1-propanol with PCC in dry CH2Cl2 provides propionaldehyde. A side product can be the ester, n-propylpropionate (CH3CH2CO2CH2CH2CH3). Explain and illustrate. [ORGO, alcohols, #3. What if water were present?]
3. Reactions: (40 pts.; equal weight) Identify the unknown structures and
answer questions in 4 of 6 of the
following problems. Explain
briefly. If you do more than four problems, cross out the ones that you do not
want graded.

Écontinued
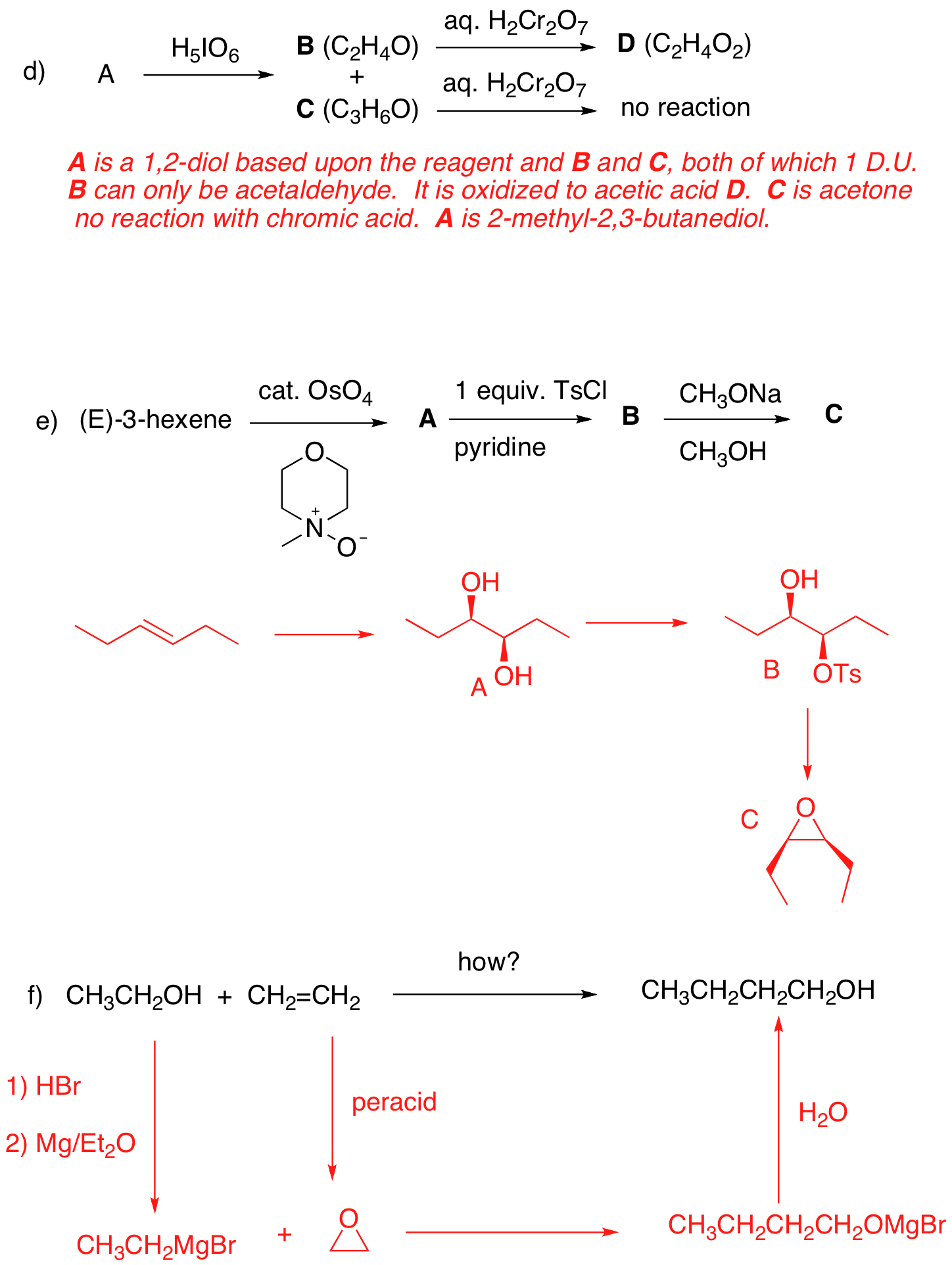
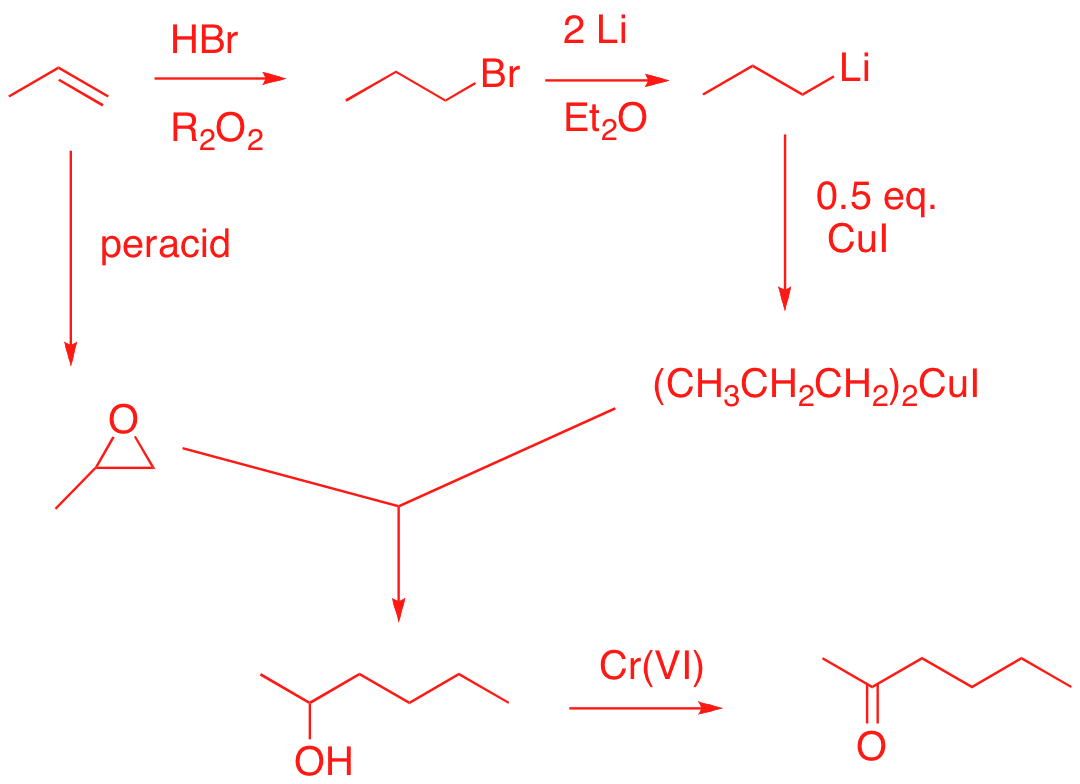
4. Synthesis: (20 pts.) Design a synthesis of 2-hexanone using propene as your only source of carbon. All reagents are available to you.
Periodic Table
Work Sheet
Work Sheet
Work Sheet