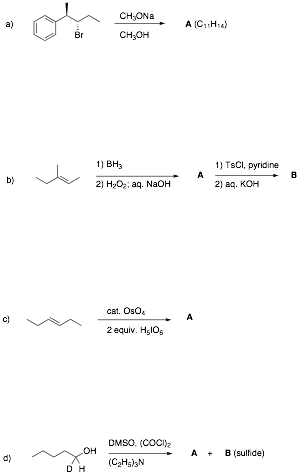
1. Reactions I: (6 x 8 pts. = 48
pts.) Complete 6 of 8 of the following
questions. Pay attention to stereochemistry,
etc. Identify the unknown structures and
answer any questions. Give very
brief explanations for your answers.
If you do more than six
questions, cross out the ones that you do not want graded.
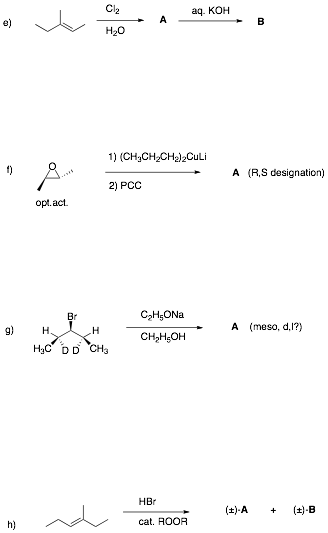
Écontinued
2) Structure: (20 pts.) Optically inactive compound A (C10H20) reacts with cat. OsO4/NMMO (N-methylmorpholine N-oxide) to produce a single, racemic compound B. Periodic acid reacts with B to form a single racemic compound C (C5H10O).
a) (15 pts.) What are the structures of A – C? Explain your reasoning.
b) (5 pts.) Compound A does not have a plane of symmetry, yet it is optically inactive? Explain and illustrate.
3) Structure/Conformation: (30 pts.) The monoterpene d-neomenthol [(1S, 2S, 5R)-2-isopropyl-5-methyl-1-cyclohexanol] has a specific rotation of [a]D = 160.
a) (5 pts.) Draw d-neomenthol (flat structure, bold and dotted bonds).
b) (5 pts.) Draw the chair conformations of d-neomenthol on the appropriate sides of the equilibrium arrows.
c) (10 pts.) The tosylate of d-neomenthol reacts with C2H5ONa/ C2H5OH to form a single optically active compound A (C10H18). Catalytic hydrogenation of A forms a single, optically inactive compound B. What are the structures of A and B? Explain and illustrate.
d) (10 pts.) A sample of d-neomenthol contaminated with its enantiomer has an observed rotation of +4 degrees. What is the optical purity of the sample and what fraction of each enantiomer is present? Show work.
4) Potpourri: (8 x 6 pts. = 48 pts.) Complete 8 of 10 of the following questions. If
you do more than eight questions, cross out the ones that you do not want
graded.
a) Circle the species that have sp2 hybridized atoms.
acetone [BeCl3]- CO2 BCl3 acetylene
b) Why does increased alkyl substitution of a double bond stabilize the alkene relative to some less substituted constitutional isomer? Be brief.
c) Circle the compound(s) that have a Degree of Unsaturation (D.U.) of 5.
C12H18N2Cl2 C10H17BrOS C11H15NO
C18H30BrClN4 C8H9NCl2
d) Estimate the heat of formation (kcal/mol) of n-octane given that the heat of formation of n-butane is -30 kcal/mol. Show work.
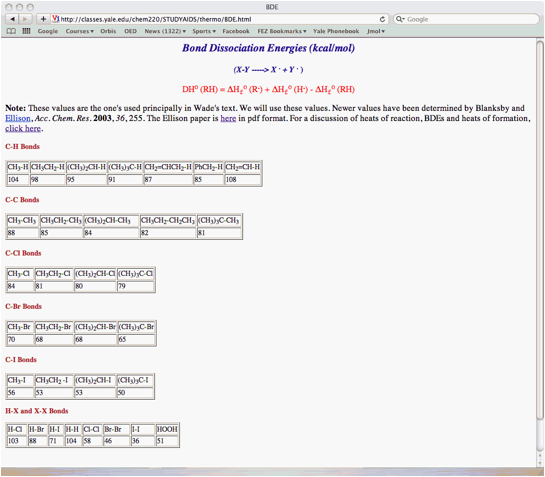
e) Determine the heat of formation of an ethyl radical (kcal/mol) given the data in 4d and in the BDE table (pg. 13). [A Standard State diagram will help.]
Écontinued
f) Circle the acids that are readily deprotonated by methyl magnesium bromide.
(CH3) 2NH C2H2 ethanol CH4S phenol
g) Rank the following acids in order of increasing acidity (1 ˆ 5; least ˆ most).
PhOH CH3CH2CO2H CH3CHClCO2H CH3CHFCO2H ClCH2CH2CO2H
_____ _________ _________ _________ _________
h) Provide an example and briefly explain and/or illustrate how an anti-bonding molecular orbital plays a role in some organic chemical process discussed this term.
i) Circle the compounds that have net molecular dipoles.
1,3-dichloro-1,2-propadiene propane (E)-dibromoethylene
1,2-dibromoethane chloroform
j) Draw the possible constitutional isomers and diastereomers of dichlorocyclobutane arising from the free radical chlorination of chlorocyclobutane.
5) SN2/E2 Kinetics:
(4 x 8 pts. = 32 pts.) Complete 4
of 6 of the following questions. If
you do more than four questions, cross out the ones that you do not want
graded.
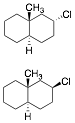
a) Circle the compound that reacts faster with CH3ONa/CH3OH
in an E2 reaction. Illustrate and explain briefly.
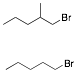
b) Circle the bromide that gives the larger E2/SN2 ratio of
products when exposed to C2H5ONa/C2H5OH.
Explain briefly.
c) Circle the compound that undergoes E2 elimination more slowly. What is the product of that reaction? Explain briefly.
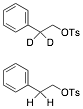
Écontinued
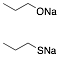
d) Given one equivalent of each of the sodium salts below, what is the expected product upon reaction with one equivalent 1-iodopropane? Explain briefly.
e) In the SN2 reaction of
ethyl tosylate with LiCl, will the reaction be faster in methanol or
dimethylformamide (DMF) as a solvent? Explain briefly.
f) In the reaction of
2-bromo-2-methylbutane with sodium methoxide or potassium tertiary butoxide,
which base will give the greater ratio of Zaitsev/Hofmann product? Explain and illustrate.
6) Thermochemistry: (30 pts. total) Create a Standard State diagram for this problem on the back of page 9 (to your left). The diagram is worth 5 points.
a) (5 pts.) Given that the hydrogenation of an alkene is exothermic, assign the heats of formation -14.8, -15.2 and -41.0 kcal/mol to 3-methylpentane, (E)- and (Z)-3-methyl-2-pentene. Explain briefly.
b) (10 pts.) Given the heats of
formation of CO2 and H2O as -94.0 and -68.3 kcal/mol,
respectively, determine the heat of combustion of 3-methylpentane. Show
work.
c) (5 pts.) Determine the heats of
hydrogenation of (E)- and (Z)-3-methyl-2-pentene. Show
work.
d) (5 pts.) Determine the heat of combustion of (Z)-3-methyl-2-pentene without using the heat of formation of CO2. Show work.
7) Synthesis: (20 pts.) Design a synthesis of
2-methylcyclohexanone. Only methane
and cyclohexane are available as the sources of carbon for the target molecule. All organic and inorganic reagents are
available to you.
8) Thermochemistry: (30 pts.)
a) (20 pts.) Provide the two propagation steps for the free radical monochlorination of 2,2-dimethylpropane (neopentane) using the bond dissociation energies in the BDE Table (pg. 13). Place the reactants and products in the appropriate boxes and the BDEs on the lines. Calculate the heat of each propagation step and the heat of the overall reaction.
b) (10 pts.) Calculate the heat of formation of 1-chloro-2,2-dimethylpropane. Data: DHfo (kcal/mol): neopentane, -40.2; HCl, - 22.1.
Periodic Table
Work Sheets
Work Sheets