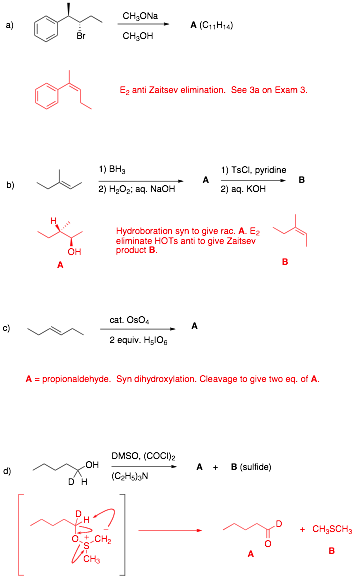
1. Reactions I: (6 x 8 pts. = 48
pts.) Complete 6 of 8 of the following
questions. Pay attention to stereochemistry,
etc. Identify the unknown structures and
answer any questions. Give very
brief explanations for your answers.
If you do more than six
questions, cross out the ones that you do not want graded.
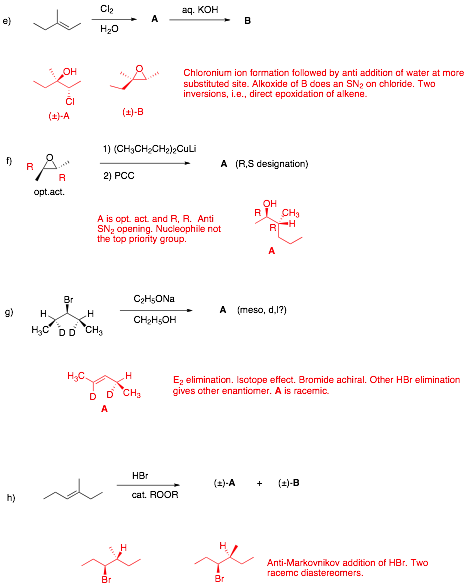
 Écontinued
Écontinued
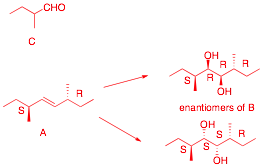
2) Structure: (20 pts.) Optically inactive compound A (C10H20) reacts with cat. OsO4/NMMO (N-methylmorpholine N-oxide) to produce a single, racemic compound B. Periodic acid reacts with B to form a single racemic compound C (C5H10O).
a) (15 pts.) What are the structures of A – C? Explain your reasoning.
A has 1 DU. Must be a
double bond because of reactions. C
has 1 DU. No
C5 ketone can be a racemate. Not enough carbons to make an
asymmetric carbon. C can only be
2-methylbutyraldehyde. We have (R)-C and (S)-C to give (±)-C. But is
the double bond cis or trans? It is trans as shown below. The Z-isomer would give B and BÕ as SRSR
and SSRR, which are diastereomers.
b) (5 pts.) Compound A does not have a plane of symmetry, yet it is optically inactive? Explain and illustrate.
A has a center of symmetry
just like the anti conformation of meso-tartaric acid.
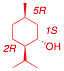
3) Structure/Conformation: (30 pts.) The monoterpene
d-neomenthol [(1S, 2S,
5R)-2-isopropyl-5-methyl-1-cyclohexanol] has a specific rotation of [a]D
= 160.
[The configuration on the exam was mistakenly given as (1S,
2R, 5R), which is actually d-isomenthol. The answers to c and d were not
affected. The answers here are given as d-isomenthol.]
a) (5 pts.) Draw d-neomenthol (flat structure, bold and dotted bonds).
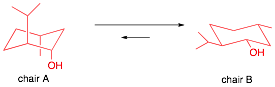
b) (5 pts.) Draw the chair conformations of d-neomenthol on the appropriate sides of the equilibrium arrows.
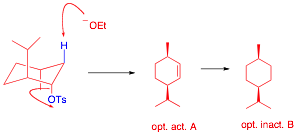
c) (10 pts.) The tosylate of d-neomenthol reacts with C2H5ONa/
C2H5OH to form a single
optically active compound A (C10H18). Catalytic hydrogenation of A forms a single, optically inactive compound B. What are the
structures of A and B?
Explain and illustrate. Only the chair A tosylate has an axial b-H and vicinal axial leaving group. Alkene A is opt. act. since the menthol was optically
active. Hydrogenation of A creates a plane of symmetry in B.
d) (10 pts.) A sample of d-neomenthol contaminated with its enantiomer has an observed rotation of +4 degrees. What is the optical purity of the sample and what fraction of each enantiomer is present? Show work. o.p. = ee = 4/16 = 0.25 or 25%. nd + nl = 100; nd - nl = 25; nd = 62.5%, nl = 37.5% or nd = 5/8, nl = 3/8.
4) Potpourri: (8 x 6 pts. = 48 pts.) Complete 8 of 10 of the following questions. If
you do more than eight questions, cross out the ones that you do not want
graded.
a) Circle the species that have sp2 hybridized atoms.
acetone [BeCl3]- CO2 BCl3 acetylene
b) Why does increased alkyl substitution
of a double bond stabilize the alkene relative to some less substituted
constitutional isomer? Be brief. C-H bonds of alkyl
groups donate electrons to anti-bonding pi bond.
c) Circle the compound(s) that have a Degree of Unsaturation (D.U.) of 5.
C12H18N2Cl2 C10H17BrOS C11H15NO
C18H30BrClN4 C8H9NCl2
d) Estimate the heat of formation (kcal/mol) of n-octane given that the heat of formation of n-butane is -30 kcal/mol. Show work. Each increase in a CH2 group in a homologous series makes the heat of formation more negative by 5 kcal/mol. So, the difference between n-butane and n-octane is 4 CH2Õs or -02 kcal/cal. Tus -20 + (-30) = -50 kcal/mol.
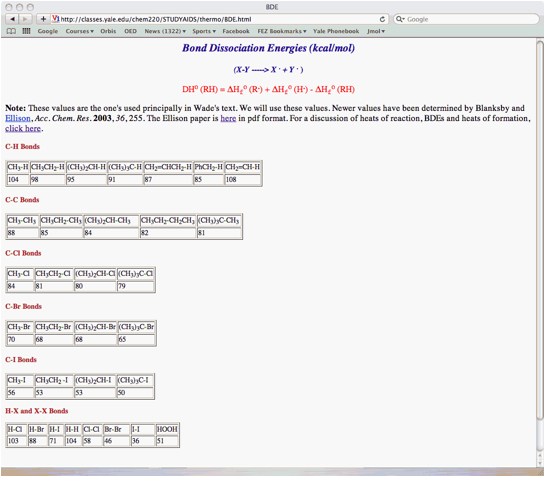
e) Determine the heat of formation of an ethyl radical (kcal/mol) given the data in 4d and in the BDE table (pg. 13). [A Standard State diagram will help.]

Écontinued
f) Circle the acids that are readily
deprotonated by methyl magnesium bromide. The Grignard reagent is the conjugate base of methane pKa = ~
50, the weakest acid (strongest conjugate base) on our table.
(CH3)
2NH
C2H2
ethanol
CH4S
phenol
g) Rank the following acids in order of increasing acidity (1 ˆ 5; least ˆ most).
PhOH CH3CH2CO2H CH3CHClCO2H CH3CHFCO2H ClCH2CH2CO2H
__1___
____2_____
____4_____
___5______
___3______
h) Provide an example and briefly explain and/or illustrate how an anti-bonding molecular orbital plays a role in some organic chemical process discussed this term.
Why more alkyl substitution of double bonds gives lower heat
of formation, i.e., stability.
Hyperconjugation: Donation of bonding C-H sp3 hybrid into anti-bonding
pi orbital of double bond.
Formation of a Grignard reagent: Addition of electron into
antibonding sigma MO.
Reductive formation of 2,3-dimethyl-2,3-butanediol
by active metal reduction of acetone.
Add electron to antibonding pi orbital (radical anion).
i) Circle the compounds that have net molecular dipoles.
1,3-dichloro-1,2-propadiene propane (E)-dibromoethylene
1,2-dibromoethane
chloroform
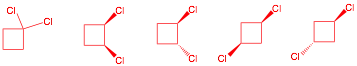
j) Draw the possible constitutional
isomers and diastereomers of dichlorocyclobutane arising from the free radical
chlorination of chlorocyclobutane. All compounds are
achiral or racemic.
5) SN2/E2 Kinetics:
(4 x 8 pts. = 32 pts.) Complete 4
of 6 of the following questions. If
you do more than four questions, cross out the ones that you do not want
graded.
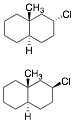
a) Circle the compound that reacts faster with CH3ONa/CH3OH
in an E2 reaction. Illustrate and explain briefly. Trans decalins do not undergo chair-chair interconversion. In
both structures the ring fusion methyl and hydrogen are both axial to both
rings. The chlorine in the lower structure is axial,
the optimal conformation for anti E2 elimination. You are not asked for the products, just
which one reacts faster, which is the lower structure.
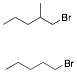
b) Circle the bromide that gives the larger E2/SN2 ratio of
products when exposed to C2H5ONa/C2H5OH.
Explain briefly. The top structure
with alpha-branching will repress SN2 reaction. It will have the higher ratio.
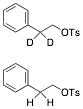
c) Circle the compound that undergoes E2 elimination more slowly. What is the product of that reaction? Explain briefly. C-D bonds react more slowly in E2 reaction. The
upper structure will eliminate more slowly.
Écontinued
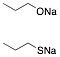
d) Given one equivalent of each of
the sodium salts below, what is the expected product upon reaction with one
equivalent 1-iodopropane? Explain briefly. The thiol anion is the better nucleophile. It
will react faster than the alkoxide in an SN2 reaction competing for a limited
amount of halide. The product is d-n-propyl sulfide.
e) In the SN2 reaction of
ethyl tosylate with LiCl, will the reaction be faster in methanol or
dimethylformamide (DMF) as a solvent? Explain briefly. The reaction is faster in the polar, aprotic solvent
DMF. Chloride ion is solvated
(hydrogen bonding)by methanol which leaves chloride
less nucleophilic.
f) In the reaction of
2-bromo-2-methylbutane with sodium methoxide or potassium tertiary butoxide,
which base will give the greater ratio of Zaitsev/Hofmann product? Explain and illustrate. This example was covered in class. The less hindered base
CH3ONa gives the higher ratio.
6) Thermochemistry: (30 pts. total) Create a Standard State diagram for this problem on the back of page 9 (to your left). The diagram is worth 5 points.
a) (5 pts.) Given that the
hydrogenation of an alkene is exothermic, assign the heats of formation -14.8,
-15.2 and -41.0 kcal/mol to 3-methylpentane, (E)- and (Z)-3-methyl-2-pentene.
Explain briefly. Because hy drogenation is exothermic, 3-methylpentane must be
-41.0. The E-isomers is more stable than the Z-isomer. E = -15.2; Z = -14.8.
b) (10 pts.) Given the heats of
formation of CO2 and H2O as -94.0 and -68.3 kcal/mol,
respectively, determine the heat of combustion of 3-methylpentane. Show
work. C6H14.= [ 6x(-94.0)
]+[14x(-68.3)] – 41.0 =
-1479.2 kcal/mol.
c) (5 pts.) Determine the heats of
hydrogenation of (E)- and (Z)-3-methyl-2-pentene. Show
work.
E = -41.0 – (-15.2) = -25.8 kcal/mol
Z = -41.0 – (-14.8) = -26.2 kcal/mol
d) (5 pts.) Determine the heat of
combustion of (Z)-3-methyl-2-pentene without using the heat of formation of
CO2. Show work. In part b, add -41 to -1479.2 to get the value -1520.2 for
the heat of combustion of 6C + 7H2O.
But combustion of the Z-alkene yields one less mole of H2O. Therefore 6C + 6H2O = -1520.2
–(-68.3)= -1451.9. Subtract
-14.8 for the heat of formation of the Z isomer gives -1437.1 kcal/mol.
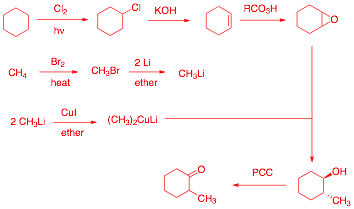
7) Synthesis: (20 pts.) Design a synthesis of
2-methylcyclohexanone. Only methane
and cyclohexane are available as the sources of carbon for the target
molecule. All organic and inorganic
reagents are available to you. This is the best route. The epoxidation may also be
accomplished by halohydrin formation followed by treatment with base.
Chlorination of methane or bromination of cyclohexane is also acceptable. Other
chromium oxidants or Swern oxidation are OK.
8) Thermochemistry: (30 pts.)
a) (20
pts.) Provide the two propagation steps for the free radical monochlorination
of 2,2-dimethylpropane (neopentane) using the bond dissociation energies in the
BDE Table (pg. 13). Place the reactants and products in
the appropriate boxes and the BDEs on the lines. Calculate the heat of each
propagation step and the heat of the overall reaction. The BDEs in the table are slightly different
from those used during the term.
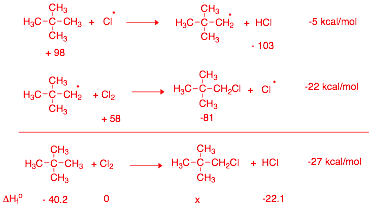
b) (10 pts.) Calculate the heat of formation of 1-chloro-2,2-dimethylpropane. Data: DHfo (kcal/mol): neopentane, -40.2; HCl, - 22.1.
DHfo
(rxn) = Hfo (products) - Hfo (reactants)
-27 = (x -22.1) – (-40.2 + 0)
-27 = x -22.1 + 40.2
-27 = x +18.1
x = Hfo
(RCl) = -45.1 kcal/mol
Periodic Table
Work Sheets
Work Sheets