EXAM 3
CHEMISTRY 220
Friday, November 12, 2010
NAME (print): ________________________________________________________
TA:_________________ Sect. Day:_________________ Sect. Time:_____________
Take a few moments to look over the exam. Answer each question on the exam paper.
No calculators or electronic devices. You may use molecular models. Important clues and structures are in bold. There is a Periodic Table on page 8.
Do all preliminary drawing or computations on the work sheets at the end of the exam. The work sheets will not be graded. You may detach the work sheets from the exam.
The exam is 55 minutes.
STOP writing and hand in your exam when you are asked to do so.
Remember: Neatness is to your advantage.
1. (15 pts) Structure Determination ______
2. (30 pts) Reactions (Do 5 of 6) ______
3. (25 pts) Potpourri (Do 5 of 6) ______
4. (30 pts) Mechanism ______
____________________________________________________
Total (100 pts)
1) Structure Determination: (15 pts.)
Optically-active compound A (C10H16)
[Degrees
of Unsaturation?] reacts with Pt/H2 to afford optically-active compound B (C10H20) [Degrees of Unsaturation?]. Ozonolysis of A followed by dimethyl sulfide reduction provides a single compound (S)-1. Draw (S)-1. What
are the structures of A and B? Explain
your reasoning.
![]()
2) Reactions: (5 x 6
pts. = 30 pts.) Complete 5 of 6
of the following questions by providing structures, reagents, etc. Pay
attention to stereochemistry, etc. Several steps may be required. If you do more than 5 questions, clearly cross out the answer
you do not want graded.
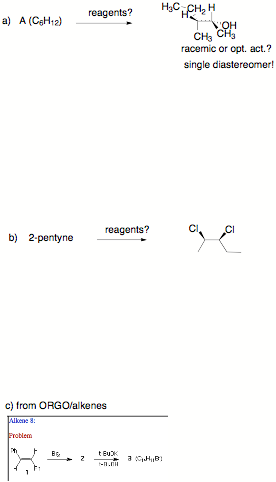
Écontinued

3) Potpourri: (5 x 5 pts. = 25 pts.) Provide the missing
information in 5 of 6 of the
following questions. If
you do more than 5 questions, clearly cross out the answer you do not want
graded.
a) Circle the terms that best apply to the hydration of 3-decyne with HgSO4/aq. H2SO4.
regioselective stoichiometric Hg++ oxidation reduction enol tautomerization
b) Circle the heptene with the greater heat of hydrogenation.

c) Circle the compound(s) with a degree of unsaturation = 9.
C17H21NO4 C17H19NO3 C17H17Cl2N
(cocaine) (morphine) (Sertraline – Zoloft)
d) Circle the terms that apply to the reaction of OsO4/H2O2 with (E)-3-hexene.
catalytic regioselective d,l-pair reduction optically-active
e) Estimate the heat of formation of 5-decyne given that DHfo = +34.8 kcal/mol for 2-butyne. Show work.
f) Write a brief description of the relevance of the work of Paul Sabatier (Nobel Prize 1912) to the chemistry covered on this exam.
4) Mechanisms: (5 x 5 pts. = 25 pts.) Complete each of the
questions below.
On Hydroboration:
i) Hydroboration is said to be an
anti-Markovnikov addition of water to a trisubstituted alkene. What critical mechanistic issue is
missing from the previous sentence? Think mechanism.
ii) The hydroboration step is a Markovnikov
addition of borane to an alkene.
It is also a reduction.
Explain.
iii) In the
second step of a hydroboration reaction, what is the reagent and what special
mechanistic feature is observed.
Écontinued
iv) In the reaction of a terminal alkyne in
a typical hydroboration procedure, why
is diisoamylborane employed rather than borane? What is the downside?
v) For the
preparation of secondary alcohols the hydroboration of the stereoisomers of
3-hexene is an acceptable procedure.
The same cannot be said for the 2-hexenes. Explain.


Work Sheets
Work Sheets
Work Sheets