EXAM 2
CHEMISTRY 220
Friday, October 15, 2010
NAME (print): ________________________________________________________
TA:_________________ Sect. Day:_________________ Sect. Time:_____________
Take a few moments to look over the exam. Answer each question on the exam paper.
No calculators or electronic devices. You may use molecular models. Important clues and structures are in bold. There is a Periodic Table on page 10.
Do all preliminary drawing or computations on the work sheets at the end of the exam. The work sheets will not be graded. You may detach the work sheets from the exam.
The exam is 55 minutes.
STOP writing and hand in your exam when you are asked to do so.
Remember: Neatness is to your advantage.
1. (30 pts) Reactions I (Do 5 of 6) ______
2. (24 pts) Reactions II (Do 4 of 5) ______
3. (22 pts) Thermochemistry ______
4. (24 pts) Potpourri (Do 4 of 5) ______
____________________________________________________
Total (100 pts)
1) Reactions I: (30 pts.;
equal weight) Provide the structures of the product(s) in 5 of 6 of the following reactions. Answer any queries that
are posed, (e. g.; opt. act.?). Very brief commentary is welcome. If
you do more than five questions, cross out the one that you do not want graded.
Pay attention to
stereochemistry, mechanisms and optical activity!
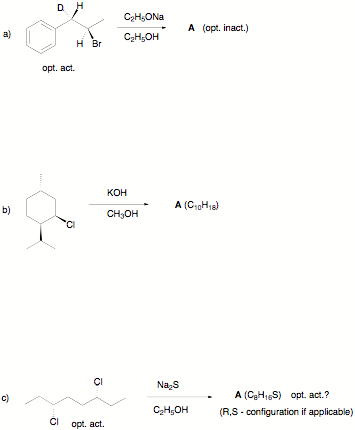
Écontinued
2) Reactions II: (24 pts., equal
weight) Answer 4 of 5 of the
following questions. Be
succinct! If you do more than four questions, cross out the one that you do not
want graded.
a) (R)-1-Bromo-2-methylbutane (1) has been reported to give a 2/3 ratio (SN2/E2)
of products upon treatment with sodium ethoxide in ethanol. What percentage of SN2 product might you expect when the
reaction is conducted with (±)-1? Explain briefly. Draw the structures of the reaction products
derived from (R)-1.
b) Will potassium tertiary butoxide
[(CH3)3COK] or sodium methoxide give a higher SN2/E2
ratio upon reaction with
1-bromopentane? Explain and illustrate briefly.
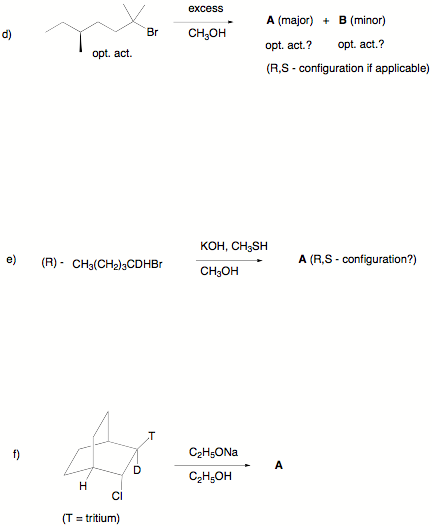
Écontinued
c) Circle the compound that will remain in excess when a
limited amount (less than stoichiometric) of KOH in ethanol reacts with equal
amounts of 1 and 2. Draw the structures of the two possible chlorine-free, non-oxygen
containing products. Give
brief explanations.
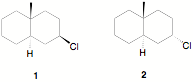
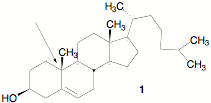
d) Cholesterol 1 is the
enantiomer shown below. Excluding the geometry of the double bond, how many
stereoisomers of cholesterol are possible? Show work. Provide
the CIP designation for the quaternary carbon designated by the arrow.
Écontinued
e) Structure 1 has a plane of symmetry and must be optically inactive. But yet structure 1 must be an eclipsed comformation which is high in energy and therefore not well-populated. Name structure 1. Assign R,S centers where needed. Why is structure 1 optically inactive in spite of the eclipsed conformation?
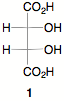
3) Thermochemistry: (22 pts.) Problem Set 3 directed you to the alkane
module of Organic Reactions Go Online (ORGO). Here is the solution to #2. Answer the three underlined questions under a), b) and
c). For part b you will also be asked
to compute the heats of the two propagation steps and the heat of the overall
reaction.
a) (5 pts.) The initiation step?
Écontinued
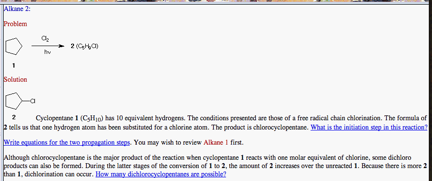
b) (12 pts.) Provide the two
propagation steps and the overall reaction for this process. Place the reactants and products in the
appropriate boxes. Compute the heats of each reaction using the data in
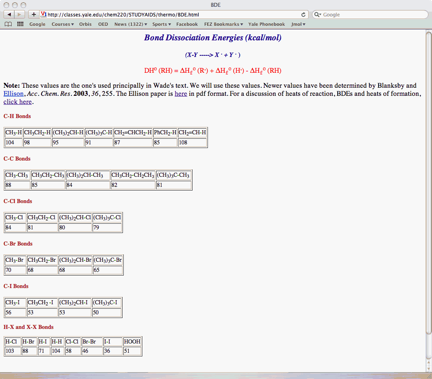
the BDE Table (pg. 10)
c) (5 pts.) How many dichlorocyclopentanes are possible?. Draw them with the aid of the templates below. No need to worry about enantiomers.
![]()
4) Potpourri: (20 pts.) Do 4 of 5 questions.
You may do either b) or d).
If you do more than four questions, cross out the one that you do not
want graded, either b) or d).
a) One of these enantiomers is not like the others. Circle it and provide its R,S – configuration at the relevant centers. Is it dextro- or levorotatory?
![]()
b) Natural tartaric acid has a specific rotation of [a] = +12o. What is the observed specific rotation of a solution containing a 2/1 ratio of natural and meso-tartaric acid, respectively? Show reasoning.
c) Circle the terms that apply to E2 reactions.
Isotope effect 1st order reaction stepwise rehybridization intermediate
d) A 3:1 mixture of (S)- and (R)-carvone,
respectively, has an optical rotation of +36o. What is the rotation of the pure (S)-enantiomer? The (R)-enantiomer?
Show work.
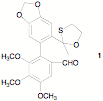
e) In 1978 racemic 1 was synthesized and was found to be a mixture of two racemic diastereomers. The method used was similar to the methodology of the 2010 Nobel Prize Medalists, only copper was used instead of palladium. i) What C-C bond was formed? ii) Why is structure 1 a mixture of two racemates?
Work Sheets
Work Sheets
Work Sheets