_____________________________________________________________________________
Total (222 pts)
Note: The final exam is worth 160 points toward the final grade.
The grade shown above will be multiplied by 0.72 to obtain the adjusted score
(222 x 0.72 = 160).
1. Spectroscopy/Structure :
(25 pts.) Achiral compound A (M+
= 112; Degrees of Unsaturation = 2) is inert to aqueous
chromic acid and its infrared spectrum displays a strong absorption at 1717 cm-1.
Compound A reacts with one equivalent of Grignard reagent B
(RMgBr) to form a mixture of achiral diastereomers C and CÕ, each of whose mass spectrum
displays a molecular ion at M+ = 128. Compound C is
more stable than compound CÕ.
Compounds C and CÕ, together or separately, react
readily with catalytic H2SO4
by an E1 mechanism to give two isomers [(±)-D (major) and achiral E (minor)]. Ozonolysis
and dimethyl sulfide reduction of compound (±)-D provides F, which upon reduction with LiAlH4 gives G, 3-methylheptan-1,6-diol. Exposure of E to excess hot aqueous KMnO4 gives rise to compounds A and H. What are the
structures of A – H? Show
your reasoning. [Hint: Work
backwards from G.]
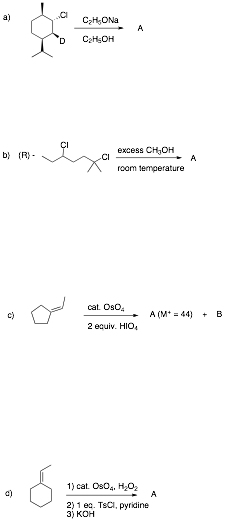
2. Reactions I: (4 x 8 pts. = 32 pts.) Do 4 of 5. Provide structures for the unknown compounds. Be explicit about stereochemistry, optical activity, racemates, etc. No mechanisms required! If you do more than four problems, cross out (with a large X through the solution) the one that you do not want graded.
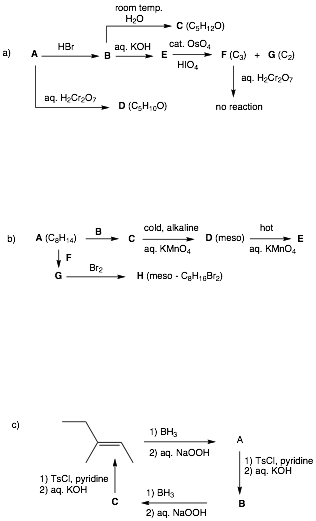
Écontinued
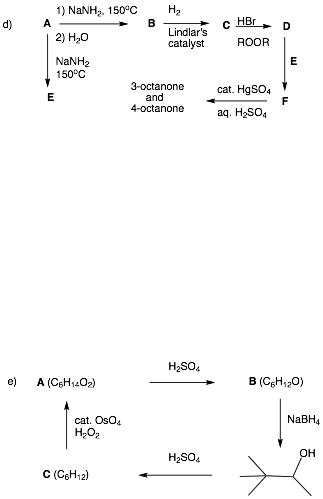
3. Potpourri: (8 x 5 pts. = 40 pts.) Do 8 of 10 problems. If you do more than eight problems, cross out (with a large X through the solution) the ones that you do not want graded.
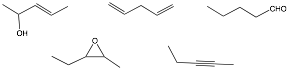
i) a) Circle the greatest number of compound(s) that are at the same oxidation level. b) Place a square around those functional groups that have sp2 hybridization.
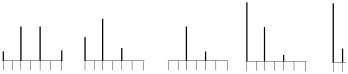
ii) Circle the mass spectral pattern that is typical of the ion CHCl2+? Explain briefly and provide relative intensities.
iii) Testosterone C19H?O2 contains four rings, one secondary alcohol, one ketone and one double bond. What is the molecular formula of this steroid? Show work.
iv) The specific rotation of (S,S)-tartaric acid is [a] = -12o. What is the percentage of each enantiomer in a mixture of the two enantiomers that has [a] = +2o. Show work.
Écontinued
v) Circle the terms that apply to E2 eliminations.
orbital overlap rehybridization isotope effect
intermediate kinetically controlled
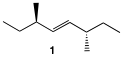
vi) (E)-(3R,6S)-3,6-dimethyl-4-octene (1) is a meso compound yet it does not have a plane of symmetry. What makes it meso? [Hint: Think of one of the two reasons why meso-tartaric acid is actually meso.]
vii) Provide a diagram and an equation that relates bond dissociation energy and heat of formation for a generic alkane, R-H.
Écontinued
viii) The bonds (shown as line bonds) in each of the following functional groups absorb energy in the infrared region. Number the structures on the lines in order of increasing vibrational frequency (1 = lowest; 5 = highest).
ix) In the free radical chlorination of (S)-1-chloro-2-methylbutane to form dichloropentanes, draw the dichloride(s) that is no longer optically active. Give a ONE WORD explanation why it is no longer optically active.
x) Circle the bases that are protonated by 1-butyne.
KNH2 RMgCl CH3ONa NH3 LiOH
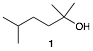
4. Synthesis: ( 25 pts.) Design a synthesis of of 2,5-dimethylhexan-2-ol (1) using 2-methyl-2-bromopropane (C4H9Br) as your only source of carbon. All other reagents are available to you. No mechanisms are required.
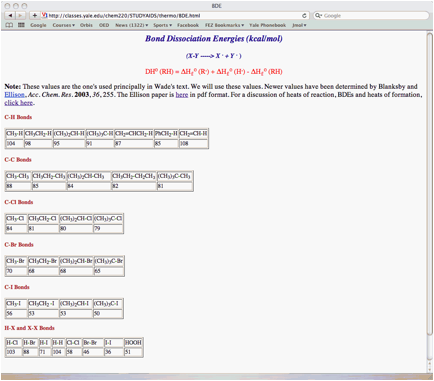
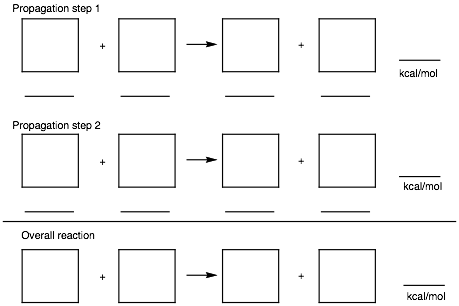
5. Thermochemistry: (25 pts.) Determine the heat of formation of chlorocyclooctane prepared by the free radical chlorination of cyclooctane. Provide the two propagation steps and the overall reaction for this process. Place the reactants and products in the appropriate boxes. Compute the heats of each reaction using the data in the BDE Table (pg. 15) and the following data: DHfo (HCl) = -22.1 kcal/mol; DHfo (cyclooctane) = -29.7 kcal/mol.
Show work.
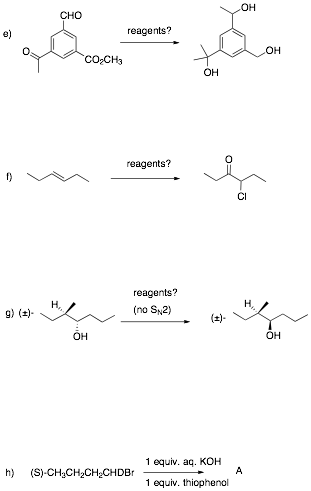
6. Reactions II: (30 pts.) Do 6 of 8. Provide structures for the unknown compounds or provide reagents. Be explicit about stereochemistry, optical activity, racemates, etc. No mechanisms required! If you do more than 6 problems, cross out (with a large X through the solution) the one(s) that you do not want graded.
Écontinued
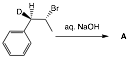
7. Kinetics/Thermodynamics: (20 pts.) Do 2 of 4. Keep your answer short and to the point! If you do more than two problems, cross out (with a large X through the solution) the one(s) that you do not want graded.
a) What is the structure of A and why? Is the reaction kinetically
or thermodynamically-controlled?
Why? Be concise.
b) The E2 reaction of
2-bromobutane with strong base (kinetically-controlled)
does not give the same distribution
of butenes (there are three of them) that is obtained
by acid-catalyzed dehydration of 2-butanol (thermodynamically-controlled). Explain briefly.
Écontinued
c) The formation of cyclohexene from 1,7-octadiene in the presence of GrubbsÕ catalyst (Ru=CHPh) and Fischer esterification illustrate two different techniques for shifting unfavorable, equilibria. Explain briefly.
d) In acyclic compounds and flexible ring compounds, why is anti-periplanar (180o dihedral angle) favored over syn-periplanar (0o dihedral angle) in E2 elimination?
8. Mechanisms: (25 pts) Provide a mechanism using the curved arrow formalism for one of the following reactions. If you do more than one problem, cross out (with a large X through the solution(s) of the one(s) that you do not want graded.
a) Swern oxidation of 2-deuterio-2-butanol
b) The conversion of 3-hexyne to (E)-3-hexene
c) The conversion of 3-hexyne to 3-hexanone in the presence f HgSO4
d) ozonolysis of cyclohexene