EXAM 3
CHEMISTRY 220a
Friday, November 13, 2009
NAME (print): ________________________________________________________
TA:_________________ Sect. Day:_________________ Sect. Time:_____________
Take a few moments to look over the exam. Answer each question on the exam paper.
No calculators. You may use molecular models. Important clues and structures are in bold.
Do all preliminary drawing or computations on the work sheets at the end of the exam. The work sheets will not be graded.
The exam is 55 minutes.
STOP writing and hand in your exam when you are asked to do so.
Remember: Neatness is to your advantage.
1. (20 pts) Reactions (Do 3 of 4) ______
2. (20 pts) Structure Determination ______
3. (20 pts) Synthesis ______
4. (20 pts) Potpourri ______
5. (20 pts) Mechanisms (Do 1 of 3) ______
___________________________________________
Total (100 pts)
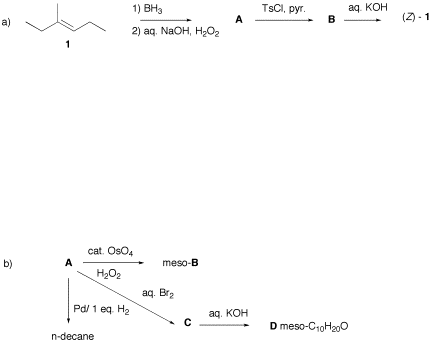
1) Reactions: (20
pts.) Do 3 of 4 of the following
questions. Identify the unknown
compounds and rationalize their formation. Pay attention to stereochemical and mechanistic issues. No
mechanisms required. If
you do more than three questions, cross out the one you do not want graded.
Écontinued
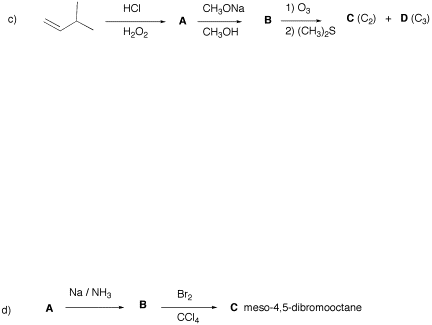
2) Structure: (20
pts) Compound A (C10H20)
undergoes ozonolysis to produce a single, optically active compound (S)-B. [At this point you should know everything but one
fact.] The reaction of compound A
with Br2 in CCl4 provides a single, optically
active compound C. What are the structures of A-C? Show their stereochemistry. Show your
reasoning. [A similar problem
appeared on PS7.]
|
3) Synthesis: (20 pts) Design a synthesis of the racemic epoxide shown on the right using 1-pentyne as your only source of carbon. Think backwards (retrosynthesis). All reagents and reactions are available to you. Show work. (Mechanisms are not required,; just reaction conditions and steereochemistry.) |
|
4) Potpourri: (20 pts.; equal weight) Answer each of the following questions.
a) Given that 1-hexyne has DHfo = +29.2 kcal/mol, what normal chain, terminal alkyne has a heat of formation of ~ 0 kcal/mol. Show work and structure/name of the alkyne.
b) Circle the terms that apply to the conversion of an alkene to an alcohol via hydroboration.
retention of configuration anti-Markovnikov addition of water
regioselective stereospecific Markovnikov addition of borane
c) Circle the reagents that add to a double bond in a syn fashion.
BH3 KMnO4 RCO3H Zn(Cu); CH2I2 OsO4
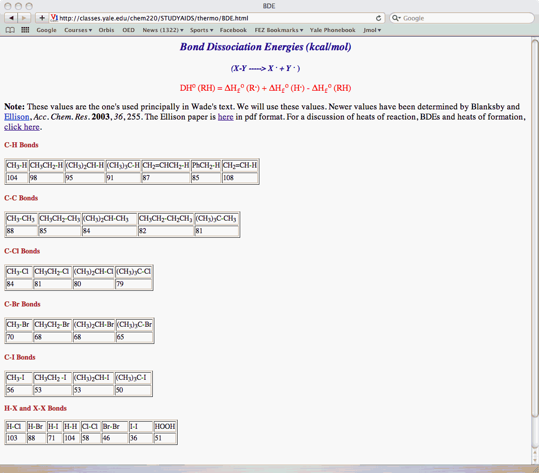
d) The BDE of the double bond in (E)-3-hexene is 144 kcal/mol (s- and p-bonds together). Using the BDE Table (page 8), show that the bromination of (E)-3-hexene has DHrxno = -28 kcal/mol. Show work.
5) Mechanisms: (20 pts.) Provide a mechanism for one and
only one of the following reactions using the curved arrow formalism. Pay
attention to stereochemistry where it applies. If you do more than one question, cross out the
one(s) you do not want graded.
a) Hydroboration of (Z)-3-methyl-3-hexene followed by oxidation to form an alcohol.
b) Ozonolysis and reduction of (Z)-3-methyl-3-hexene.
c) Mercuric ion-catalyzed hydration of 3-hexyne to form 3-hexanone.
Work
Sheets
Work Sheets
Work Sheets