FINAL EXAM
Organic Chemistry
Chemistry 220a; 2 P.M., Wednesday, December 14, 2005
NAME (print): ______________________________________________________________
TA:_____________________ Section Day:_____________ Section Time:______________
Take a few moments to look over the exam. Do problems first with which you are most comfortable. Important points and unknowns are in bold type. Do all preliminary work on the worksheets. The worksheets will not be graded. The exam is the length of two hour exams with an additional one hour for review. STOP writing when you are asked to do so. Put your name on the cover sheet and subsequent pages (except for Work Sheets) where indicated.
.Remember: Neatness is to your advantage.
Have a GREAT winter
break!
1. Spectrometry/Structure I (30 pts) ________ 5. The Standard State (25 pts) _________
2. Reactions I (32 pts; 4 of 6) _________ 6. Mechanisms (25 pts.) _________
3. Potpourri (48 pts; 6 of 8) _________ 7. Synthesis (25 pts.) _________
4. Reactions II (32 pts) _________ 8. P.M.H.S.W.W. (33 pts.) _________ ______________________________________________________________________________
Total (250 pts)
1. (30 pts) Spectrometry/Structure: Optically-active compound A, whose mass spectrum is shown below, is converted into ketone (S)-B with PCC or aqueous chromic acid. Compound A forms tosylate C, which upon treatment with aqueous KOH, produces achiral alkene D as the Zaitsev product. If tosylate C is exposed to LiBr in acetone (solvent), compound E is formed. When E is treated with aqueous KOH, achiral compound F is formed as the principal constitutional isomer. Hydrogenation of D or F produces the same alkane G but D liberates more heat (0.4 kcal/mol) than F. Warm permanganate oxidation of D or F affords H (C4H8O) and I (C2H4O2). [Hint: Working backwards is useful.]
a) (25 pts) What are the structures A-H? Explain and illustrate. Do not forget absolute and relative stereochemistry where they apply. [The structures can be determined without the spectrum.]
b) (5 pts) Explain the source of the base peak in the spectrum, i. e., why is m/z 87 not the base peak.
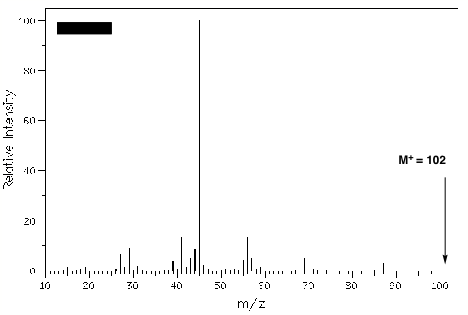
Continued on the next page...
1. Continued
2. (4 x 8 pts = 32 pts) Reactions I: Provide the reagents (and conditions, if necessary) required to complete 4 of 6 of the following chemical transformations. If additional carbon atoms are necessary, select your own source. Several steps may be required. If you complete more than four questions, cross out the ones that you do not want graded.
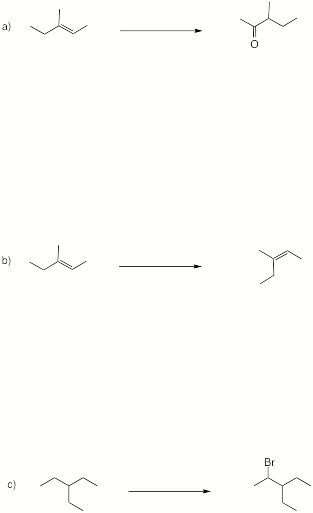
continued on the next page…
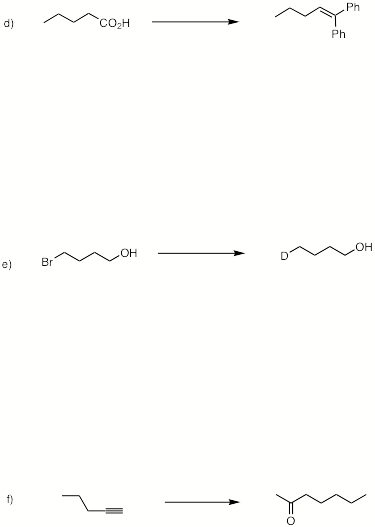
3. (6 x 8 pts = 48 pts) Potpourri: Complete 6 of 8 of the following questions. If you complete more than six questions, cross out the ones that you do not want graded.
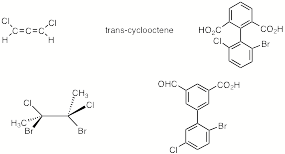
a) Circle the racemic compounds that are capable of resolution.
b) Describe fully (name, structure, etc.) the major monobromide derived from the free radical bromination of (S)-1-chloro-2-methylbutane. Briefly explain your answer.
c) For n-octane at 25oC: DHf0= -49.8 kcal/mol; DH0(combustion) = - 1,317 kcal/mol. Estimate the heats of formation and combustion for n-hexane. Show work.
d) Circle the compounds that are in violation of Bredt’s Rule.
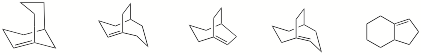
continued on the next page….
e) Circle the reactions that are stereospecific with the (E)- and (Z)-2-butenes.
bromination epoxidation osmylation hydroboration
permanganate dihydroxylation
f) Circle the compounds that are readily deprotonated by aqueous KOH.
acetic acid phenol methanethiol butyne ammonia
g) Circle the compounds that contain atoms with sp hybridization.
Vinylacetylene-(C4H4) BEt3 acetone CO2 allene
h) What is the
percentage of the minor enantiomer when a mixture of enantiomers has an optical
purity of 40%. Show work.
4. (4 x 8 pts = 32 pts) Reactions II: Complete 4 of 6 of the following questions. If you complete more than four questions, cross out the ones that you do not want graded.
Continued on the next page…
5. (25 pts) Standard State: Draw a Standard State Diagram that illustrates the heat of formation of a methyl radical and the heat of combustion of methane. In addition, calculate these two quantities from the data provided. The diagram need not be to scale. Show work. DHfo (methane) = -17.4 kcal/mol; DHocombustion(graphite) = -94.0 kcal/mol; DHocombustion(H2) = -68.3 kcal/mol; BDE (H2) = 104 kcal/mol; BDE (methane) = 104 kcal/mol.
Standard State
________________________________________________
6. (25 pts) Mechanism: One of the reactions of diols that I neglected to discuss is the pinacol rearrangement. The prototypical rearrangement is the conversion of 2,3-dimethylbutane-2,3-diol (“pinacol”, 1) into 3,3-dimethyl-2-butanone (‘pinacolone”, 2).
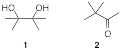
a) What is the electron change in the reaction 1 ą 2?
b) Provide a mechanism (curved arrow formalism) using the reagent that catalyzes this reaction.
c) Provide the three individual steps and reagents for the reverse reaction,---namely the conversion of 2 ą 1.
d) The answer to a) must be the same as the total electron change in c). Illustrate and explain briefly.
7. (25 pts) Synthesis: Capsaicin (1) is the “hot” in hot chili peppers. This compound has found use in pain management in medicine. To study the effect of double bond location in 1, acid 2 is required to synthesize a double bond analog of 1. A student designs a synthesis of 2 from acetylene, isobutylene, and 4-bromo-1-butanol. Her plan is to use acetylene chemistry. She also recognizes that isobutylene will not only be incorporated into carboxylic acid 2 but it will also serve as a protecting group in her planned synthesis. She has all reagents available to her. Show what she has in mind.
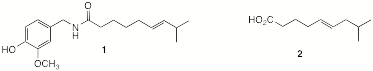
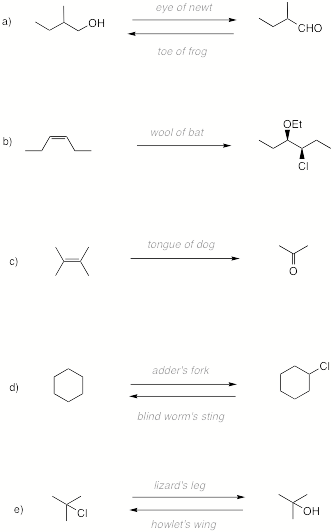
8.
(33 pts.; 4 x 8 potions; 1 pt. re: gate) P.M.H.S.W.W. “Known for tormenting students with laborious
homework assignments while cooking up sinister potions in class, Snape seems
like a textbook Orgo professor.”
"Snape's
Yale analogue is most definitely Professor Frederick Ziegler," Mark Fisher
'07 said. "Infamous for his tacit encouragement of competition among his
more elite students, so I hear, he seems the most likely candidate to have
invented high-level, malicious organic chemistry reactions in the margins of
his college text book." YDN,
11/18/05.
Alas,
my alter ego has been revealed (Potions Master of the Hogwarts
School
of Witchcraft and Wizardry). I leave you budding wizards of
Slytherin House, Gryffindor (particularly you, Potter!), Sillimania, and Branfordium (quod alii loci [sic]) to provide the correct “potions” for the
alchemical transformations on the next page. [Note:
The original potions were provided by three horrid hags, close
associates of the soon to be Thane of Cawdor.]
|
"Eye of
newt, and toe of frog, Wool of bat, and tongue of dog, Adder's fork, and blind-worm's sting, Lizard's leg, and howlet's wing,-- For a charm of powerful trouble, Like a hell-broth boil and
bubble." --From Macbeth (IV, i, 14-15) (What? No wolfsbane!) |
H. P. aka E. Z. |
persevero…
continued
on the next page…
8. Continued …(1 pt.) State something
knowledgeable about the chirality of the tetrahedra on the CRB gate.
