EXAM 3
CHEMISTRY 220a
Friday, November 4, 2005
NAME (print): _______________________________________________________________
TA:_____________________ Section Day:_________________ Section Time:__________
No Calculators! Take a few moments to look over the exam. Answer each question on the exam paper.
Important clues and structures are in bold.
Do all preliminary drawing or computations on the work sheets at the end of the exam. The work sheets will not be graded.
The exam is 55 minutes.
STOP writing and hand in your exam when you are asked to do so.
Remember: Neatness is to your advantage.
1. (20 pts) Structure ______
2. (30 pts) Reactions (5 of 6) ______
3. (25 pts) Potpourri ______
4. (25 pts) Mechanisms (do 1 of 4) ______
___________________________________________
Total (100 pts)
1. Structure: (20 pts) Treatment of compound A, C10H20, with O3 and then dimethyl sulfide provides a single, branched-chain ketone B. Exposure of A to bromine water affords (▒)-C, which, when treated with aqueous NaOH, produces meso-epoxide D. What are the structures A-D? Explain and illustrate.
2. Reactions: (30 pts) Complete 5 of 6 of the following questions. Specify the structures in each one with a brief rationale for your choice. Pay attention to stereochemistry, optical activity, etc. where they apply. . If you do six questions, cross out the one that you do not want graded.
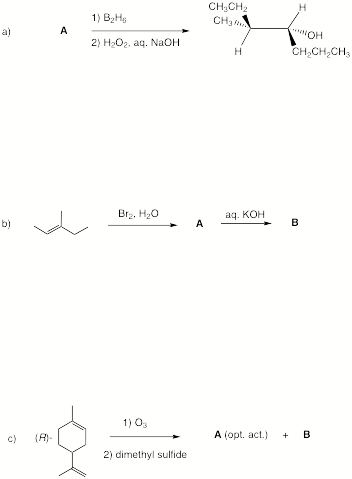
╔continued next page
2. continued.
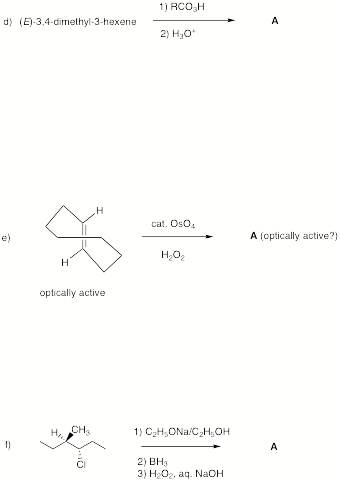
3. Potpourri:
(25 pts) Complete the following questions.
a) Circle the hexene(s) with the greatest heat of hydrogenation.
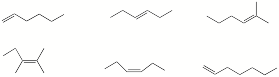
b) Circle the terms that apply to the addition of bromine to the 2-pentenes.
stereospecific regiospecific meso anti bromonium ion
c) Circle the terms that apply to the conversion of (E)- or (Z)-3-methyl-2-pentene to the alcohol 3-methyl-2-pentanol by hydroboration/NaOOH.
presence of diastereomers optically active stereospecific
common intermediate anti addition
d) Of the following additions to alkenes, circle the operations that we do not know how to accomplish.
form a 1,2-diol anti add
Br2 syn form
a chlorohydrin syn
form a 1,2-diol
syn
add chlorine anti
e) Circle the
reactions that form meso compounds with (E)-3-hexene.
Br2/CCl4 OsO4/H2O2 1)
peracid; 2) H3O+
alkaline, aq.
permanganate ozone/(CH3)
2S
4. Mechanisms: (25 pts) Provide a mechanism for one of the following reactions. Use the curved arrow formalism. Be as explicit as possible. If the mechanism
of some particular step is not known, just write the reagent. Briefly address issues of
stereospecificity, regiochemistry, stereochemistry and stoichiometry as they
may apply. If you work
on more than one solution, cross out the one that you do not want graded. Two pages are provided.
a) The
ozonolysis of (E)-2-hexene.
b) The reaction
of (Z)-2-hexene with m-chloroperbenzoic
acid and the subsequent reaction of the product with aqueous mineral acid.
c) Epoxidation
of (E)-2-hexene via the halohydrin
route.
d) Formation of
an alcohol from
(Z)-3-methyl-3-hexene by the borane method.
Continued work space on
the next page╔
4. Continued╔.
Work Sheets
Work Sheets
Work Sheets