EXAM 2
CHEMISTRY 220a
Friday, October 15, 2004
NAME (print): ___________________________________________________
TA:_____________________ Day:_________________ Time:__________
Take a few moments to look over the exam. Answer each question on the exam paper.
Important clues, points, and structures are in bold.
Do all preliminary drawing or computations on the work sheets at the end of the exam. The work sheets will not be graded.
The exam is 55 minutes.
STOP writing and hand in your exam when you are asked to do so.
Remember: Neatness is to your advantage.
1. Thermochemistry (28 pts) ______
2. Reactions (24 pts, Do 4 of 5) ______
3. Potpourri (24 pts, Do 4 of 5) ______
4. Mostly Mechanisms (24 pts, Do 4 of 5) ______
______________________________________________
Total (100 pts)
1. Thermochemistry: (28 pts.)
a)
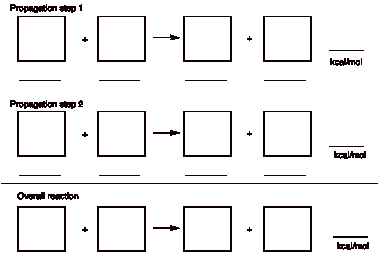
(18 pts) Provide the propagation steps and overall reaction for the gas phase,
room temperature, free radical monobromination of cycloheptane. Place
the alkane in the first box on the left.
Place the other reactants
and products in the remaining boxes with their BDEs on the appropriate
lines. See the last page 11 for
BDEs.
b) (10 pts) Determine the heat of formation of the
monobromination product in part a), given the following data: DHfo (kcal/mol):
cycloheptane (-28.2), HBr (-8.7), Br2 (+7.4), HCl (-22.1), and Cl2
(0).
2. Reactions: (4 x 6 pts. = 24 pts.) Provide reasonable structures for the products in 4 of 5 of the following reactions. If you do all five, be sure to cross out the one that you do not want graded. Comment very briefly. Pay attention to stereochemistry and absolute (R vs. S) configuration.
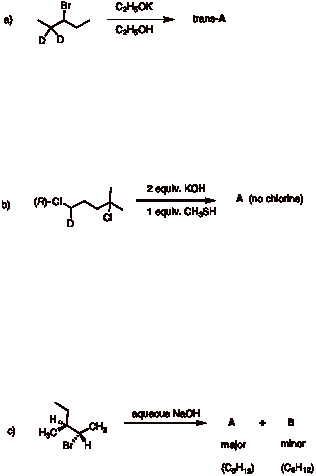
Continued on next page …
2. Continued.
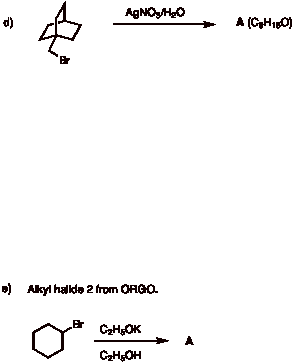
3. Potpourri: (4 x 6 pts. = 24 pts.) Do 4 of 5. If you do all five, be sure to cross out the one that you do not want graded.
a) “One of these things (Fischer projections) is not like the others. One of these things doesn’t belong.” Circle it.
![]()
b) A 3/2 mixture of enantiomers has an observed [a] = +16o. What is the best answer for the rotation of the major enantiomer? Circle it. Show work.
-80o +16o 78o +3o 0o
c) Name the following optically active by IUPAC rules.
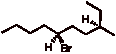
d) Free radical chlorination of (R)-1-chloro-2-methylbutane leads to five constitutional isomers, one of which has a stereoisomer present (A), one of which is achiral (B), and one of which is a racemate (C). Label A and its stereoisomer A', B, and, C below. [Note: A and A' are not distinguishable with the information provided.]
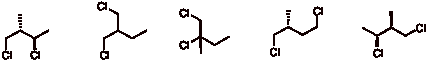
e) Why is the heat of formation of bromine given in 1b equal to its heat of vaporization? Be brief.
4. Mostly Mechanisms: (4 x 6 pts. = 24 pts.) Provide the structures or requested information in 4 of 5 of the following problems. Comment briefly. If you do all five, be sure to cross out the one that you do not want graded. [Note: Questions a-d are from the questions at the end of Chapters 5 and 6.]
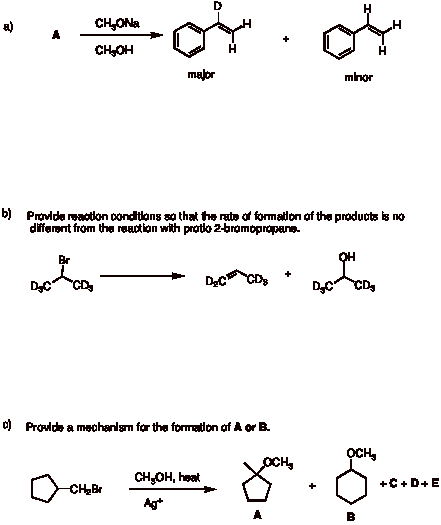
Continued on the next page …
4. Continued.
Work Sheets
Work Sheets
Work Sheets