CHEMISTRY 220a
Friday, December 7, 2001
NAME (print): ______________________________________________________________
TA:_____________________ Section Day:_________________ Section Time:__________
Take a few moments to look over the exam. Answer each question on the exam paper.
Important clues and instructions are in bold.
Do all preliminary drawing or computations on the work sheets at the end of the exam. The
work sheets will not be graded. There is a Periodic Table on the last page of the exam.
The exam is 55 minutes.
STOP writing and hand in your exam when you are asked to do so.
REMEMBER: Neatness is to your advantage.
1. (25 pts) ______
2. (25 pts) ______
3. (25 pts) ______
4. (25 pts) ______
_______________________________
Total (100 pts)
1. (5 x 5 pts. = 25 pts.) Provide the products in each of the following chemical reactions. Pay
attention to stereochemistry.

2. (5 x 5 pts. = 25 pts.) Circle the best answer in each of the following:
a) The molar ratio of CrO3 to RCH2OH required in the complete oxidation of a primary
alcohol to a carboxylic acid.
|
2/3 |
1/6 |
1/1 |
3/2 |
4/3 |
b) The compound(s) with the highest oxidation level
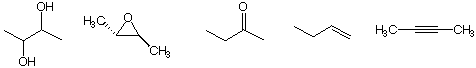
c) The operational equivalent of the reaction of an olefin with O3 followed by (CH3)2S:
|
KMnO4 cold |
KMnO4 warm |
KMnO4/ cat. OsO4 |
H2O2/cat. OsO4 |
HIO4/cat. OsO4 |
d) If a 1N solution requires 49 g of H2SO4 per liter of aqueous solution, then a 1N solution of Jones's
reagent (CrO3/aq. H2SO4 ) requires how many grams of CrO3 per liter of aqueous solution?
|
100 |
67 |
33 |
49 |
98 |
e) A chemical pathway leading from ethane to acetic acid involves a change of how many
electrons?
|
-4 |
-2 |
+4 |
-6 |
+6 |
|
3. (25 pts.) Compound A, C8H16, reacts with OsO4/H2O2 to give (±)-diol B. Compound A does NOT give n-octane upon catalytic hydrogenation. Monotosylation of B gives alcohol C, which when treated with KOH, gives meso compound D, C8H16O. Exposure of diol B to periodic acid (HIO4) provides a single compound E, C4H8O. Compound E is inert toward pyridinium chlorochromate (PCC) in CH2Cl2. However, the addition of some water to the reaction mixture affords F, C4H8O2. What are the structures A - F? Justify your answers. |
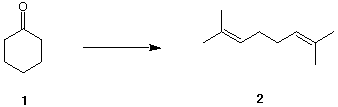
4. (25 pts.) Show how you would convert cyclohexanone 1 into 2,7-dimethyl-2,6-octadiene
2. The only other source of carbon available for the synthesis is methyl alcohol. All
reagents and carbon-based solvents are available.