Chair (larger view)
Boat (larger view)
Twist Boat (larger view)
How to manipulate JSmol structures
Cyclohexane
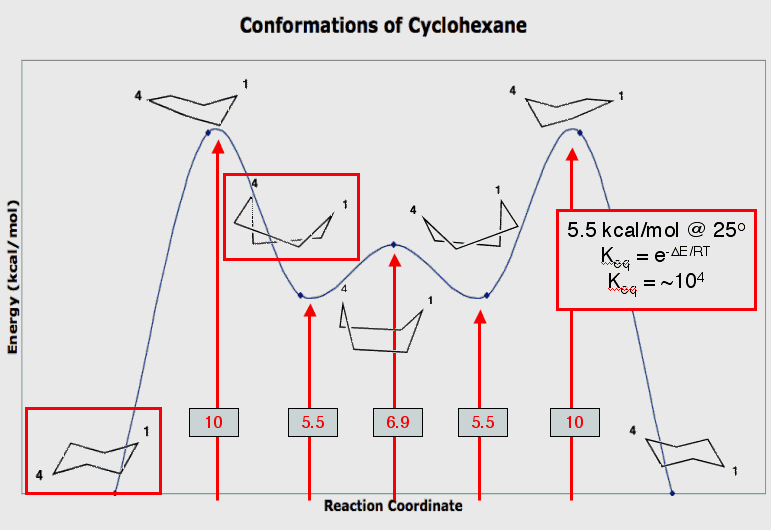
The chair is the lowest energy conformation of cyclohexane. Four carbon atoms (C2, C3, C5, and C6) lie in a plane while the other two carbon atoms (C1 and C4) lie above and below the plane. Rotate the structure and convince yourself. When the chair is viewed along any C-C bond, all the attached bonds are staggered. The C-C-C bond angles are ~111o and the H-C-H bond angles are correspondingly smaller (107o) than the ideal sp3 bond angle of 109.5o present in methane. [Measure the angles. This is the same C-C-C bond angle that is present in propane. The two terminal carbons open the angle.] In this chair cyclohexane the equatorial hydrogens are blue and the axial hydrogens are red. The boat is an unstable conformation of cyclohexane that lies 6.9 kcal/mol above the chair. Four of the carbon atoms lie in a plane with the other two carbon atoms (1,4-relationship) lying on the same side of the plane. When you view along the C-C bond bearing green hydrogens you see that the C-H bonds are eclipsed, increasing the energy of the boat. In addition, the orange hydrogens repel one another (flagpole interaction). [Measure the distance between these hydrogens. How does the value compare with the van der Waal's radius for hydrogen, 1.2 Angstroms.] The flagpole and the eclipsing interactions can be relieved by rotation about the axis that contains both violet hydrogens of the boat. Imagine holding the rear violet hydrogen in place and twist the front violet hydrogen to the left. This operation leads to the twist boat which is an energy minimum lying 5.5 kcal/mol above the chair. If you view the twist boat by superimposing the violet hydrogens, you will see that the flagpole interaction has been relieved [measure it] and that the eclipsing of the C-H bonds has been lessened but they cannot become staggered.