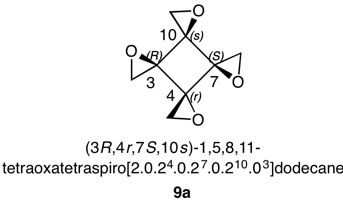
1,5,8,11-Tetraoxatetraspiro[2.0.24.0.27.0.210.03]dodecane
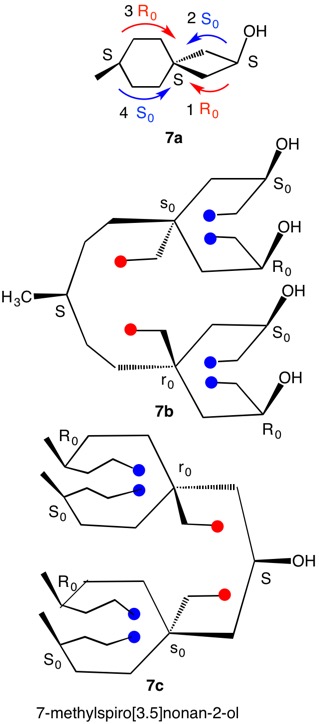
Figure 9 has three epoxide rings with the oxygens on the same side of the cyclobutane ring at C3, C4 and C10. The fourth epoxide ring at C4 has the methylene group on the same face of the cyclobutane ring as the oxygens in the other three epoxides. This compound is achiral with a plane of symmetry passing through C4 and C10. This plane will cause the configuration at C3 to be the opposite of C7. The compound is a cyclobutane analog of an inositol, which has six oxygens attached to each carbon atom of a cyclohexane. The assignment of configurations to all the inositols has been described in detail. Nonetheless, the basics will be reviewed here. Each stereocenter bears an oxygen atom and methylene group in the epoxide ring. The oxygen has the highest and the methylene the lowest priority. But how does one determine the second and third priorities? The digraphs for the four centers are on the right. Consider the top digraph. The carbon under consideration is C3 (in red). The cyclobutane carbons to the left of C3 are successively C10, C7 and C4 while to the right they are C4, C7 and C10. The vertical lines below the atom numbers indicate the location of the oxygen atoms, either above or below the plane of the ring, which is the horizontal line. To assign temporary configurations for C3, one moves successively to the left around the ring from C10 --> C7 --> C4 and, to the right, from C4 --> C7 --> C10. At each carbon the configuration is determined assuming the target atom, in this example C3, has the second priority. The descriptors for the atoms on the left of the digraph mirror their partners on the right side. But the descriptors themselves do not mirror one another! Assignments are made by 1,2 comparisons to find like pairs (Rule 4). If none is found then a 1,3-comparison and, if needed, 2,3-comparison. S0S0 is a like (lk) pair while S0R0 is unlike (ul). Now lk > ul. Therefore for C3, C10 has second priority while C4 has third priority. Carbon 3 has an R-configuration.
The configuration of C7 can be solved using the digraph but, as mentioned above, C3 and C7 are mirror images of one another. Hence, C7 is S.
The digraphs for stereotopic, achirotopic C4 and C10 display the symmetry in both atoms and descriptors. There is no possibility of a lk/ul comparison. Default is made to Rule 4, r > s (r0 > s0).
|