|
Fig. 1
|
2-Hexene A method is required to assign unambiguously the stereochemistry of double bonds. This need is fulfilled by employing the Cahn-Ingold-Prelog (CIP) Rules. The priorities of groups in 2-hexene at C2 and C3 are determined. In both examples, a carbon and a hydrogen are attached to C2 and C3. The CIP Rule 1a requires carbon, which has the higher atomic number, to have priority over hydrogen. In the case of cis-2-hexene, both carbons are on the same side of the double bond and the name (Z)-2-hexene is applied (Ger. zusammen = together). For trans-2-hexene the designation is (E)-2-hexene (Ger. entgegen = opposite). Click on "show labels". Parenthetically, when one of the carbons of the double bond has exactly the same substituents, there is no stereochemical issue and, therefore, no E/Z assignment. |
Fig. 2
|
|---|
|
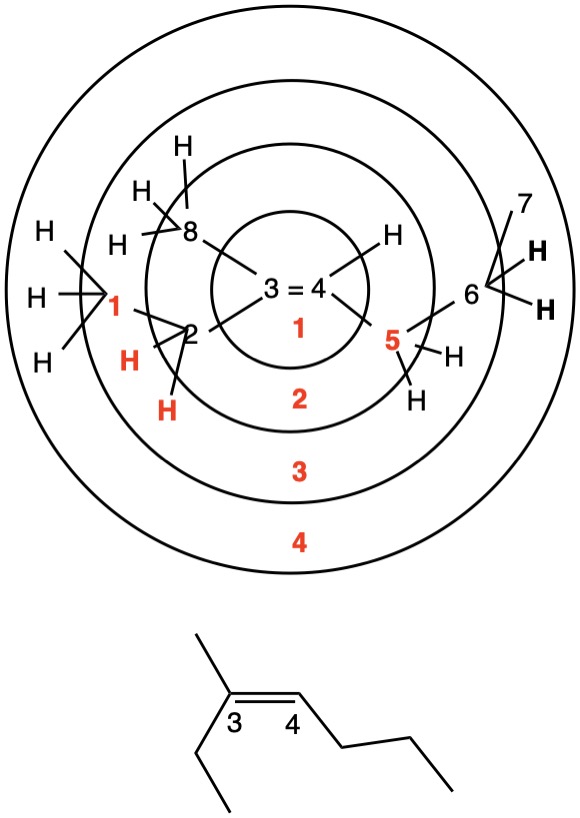
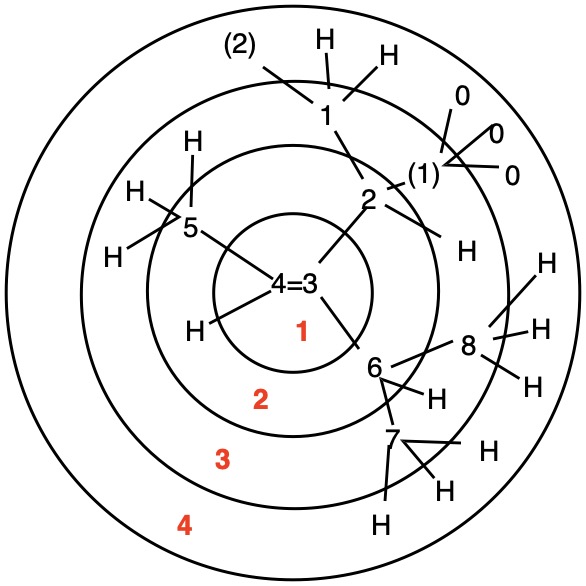
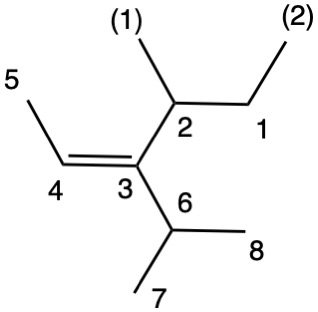
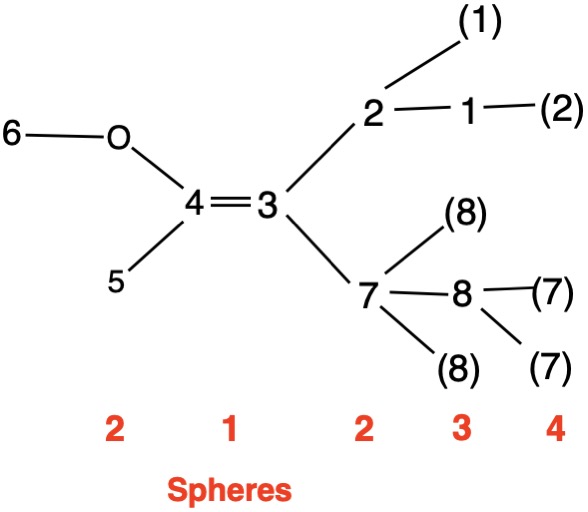
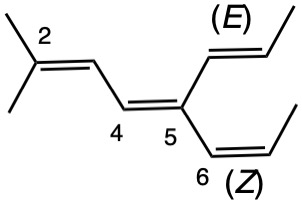
The left hand panel shows a double bond (Fig. 3a) bearing three alkyl groups. Click "show carbon #". It is apparent that at C3 ethyl>methyl and at C4 propyl>H. While such a technique is effective in this trivial example, in more complex examples it may not suffice. Digraph 3b illustrates the proper method to assess priorities. The double bond is placed in the central sphere. C3 is attached to C2 and C8 in sphere 2. In sphere 3 C2 is attached to {C1, H, H} while C8 is connected to {H, H, H}. A one-to-one comparison of these locants is made looking for the first difference. In this instance C1>H. Therefore, ethyl>methyl. The priorities for C4 are made in sphere 2 where C5>H. The double bond has the (Z)-configuration, Verify by clicking "show labels". |
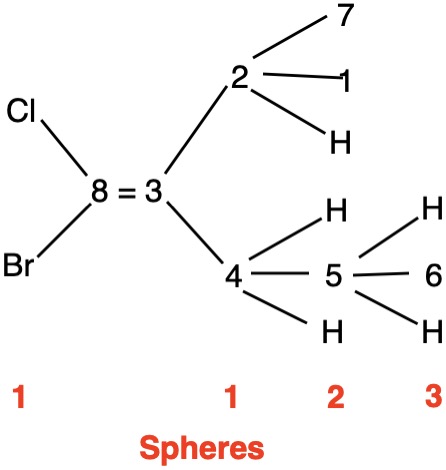 Fig. 4b |
|---|