This reaction was discovered by A.
W. Hofmann in 1851 as part of his studies that defined the amine
type. It is the elimination of a quaternary ammonium hydroxide under
thermal conditions to afford an olefin and an amine. Because the
reaction is effective at breaking carbon-nitrogen bonds, its
principle use in organic chemistry prior to the advent of
spectroscopy was as a degradative tool in the structure proof of
alkaloids. In the 20th-century mechanistic aspects of the reaction
were explored. First, we will consider this reaction as it applies to
acyclic amines, i. e., those amines in which the nitrogen atom is not
part of a ring. This does not preclude the presence of a ring in the
structure. The second class of amines to be considered are the cyclic
amines, i. e., those amines that contain nitrogen in a ring.
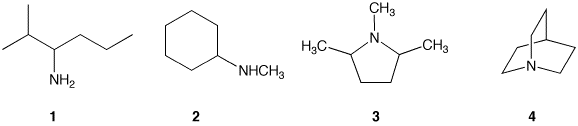
Consider the four amines shown below. Both amines 1 and
2 are acyclic in regard to nitrogen. Although amine 2
contains a ring, it is a cyclohexane ring. Both amines 3 and
4 are cyclic amines. The nitrogen in amine 3 is
contained in one ring while the nitrogen in amine 4 is
contained in two (not three) rings.

The Hofmann elimination reaction involves three distinct steps: 1) exhaustive methylation, 2) conversion of he methiodide to a an ammonium hydroxide, and 3) the actual elimination. The exhaustive methylation requires the amine to react completely with a methylating agent, usually methyl iodide, to form a quaternary methiodide salt. Thus, primary amine 1 in the presence of excess methyl iodide undergoes successive methylation to form secondary amine 5, which, in turn, is methylated to form tertiary amine 6. Finally, the quaternary methiodide 7 is formed. Three equivalents of methyl iodide were consumed in the conversion of primary amine 1 into methiodide 7. Similarly, secondary amine 2 would require two equivalents of methyl iodide while tertairy amines 3 and 4 would require only one equivalent of methyl iodide to form their respective methiodides. The number of equivalents of methyl iodide consumed provides information about the substitution pattern of the amine. Each methylation except the formation of the methiodide produces an amine hydroiodide salt. This salt must be deprotonated prior to subsequent methylation. A weak base is usually employed to this end.

The methiodide is usually converted to the hydroxide by reaction with silver oxide or by anion exchange methods. Finally, thermolysis of the quaternary ammonium hydroxide leads to elimination. If the tertiary amine and the olefin formed in the elimination are separate molecules, one can conclude that the nitrogen was not contained in a ring. If on the other hand, the olefin and the tertiary amine are contained in a single molecule, the conclusion is that the nitrogen was contained in a ring. The carbon atoms of the double bond indicate the two possible sites where the C-N bond was present. The other carbon must be the location of the b-C-H bond.

Imagine the elimination of quaternary ammonium
hydroxide 8. There are three b-C-H
bonds that may participate in the elimination. The red methine
hydrogen would lead to trisubstituted olefin 9 while the red,
diastereotopic methylene hydrogens would each lead to one of the
stereoisomeric (E)- and (Z)- olefins 10 and 11. If we did not know
the structure of 8 before hand and we isolated 8-11 and
trimethylamine, we could conclude that the nitrogen is not contained
in a ring and that only the structure 8 could afford olefins 9-11.
If, however, only olefin 9 were isolated and not 10 and 11, we could
not ascertan the position of the C-N bond in 8. A similar problem
exists for the isolation of 10 or/and 11 in the absence of 9.
The Hofmann elimination of quaternary ammonium salts, unlike the
elimination of halides, tends to give more of the less-substituted
olefin (Hofmann product) as opposed to more of the more-substituted
olefin (Saytzeff product). Both reactions have transition states that
through the anti disposition (where possible) of the
b-hydrogen
and the bulky quaternary nitrogen. Staggered conformations
12-14 are viewed along the C3-C2 bond.
all of them are candidates for transition states leading to olefin
9. Only conformation 13 has the the desired
anti-arrangement (in
red) of the hydrogen and the quaternary
ammonium group. Conformation 12 is the lowest in energy of the
three conformations having a methyl and the quaternry ammonium ion
gauche and a methyl and n-propyl group gauche. Although there is more
of conformation 12 present at equilibrium than 13 or
14, it is not conformationally suited for elimination. Both
conformations 13 and 14 have three contiguous gauche
interactions. Since the quaternary ammonium group is larger than
n-propyl which is larger than methyl, conformation 13, which
has the quaternary ammonnum group gauche to two methyl groups, is
slightly less stable than conformation. Even though conformation
13 is the minor contributor in terms of concentration, it is
the major contributor leading to olefin 9. Of the
conformations 15-17 leading to olefins 10 and
11, conformation 16 is the most stable but it is not
anti-periplanar. Conformation 15, which is more stable than
17, leads to (E)-isomer 10 while 17 would
produce (Z)-isomer 11.

Quaternization of secondary amine 2 leads
to the favored chair conformation 18a. The population of
conformation 19a, which is required for elimination, will be a minor
contributor. Consider the two related stereoisomers 18(19) b
and c. The cis isomer 18b(19b) is predicted to undergo
elimination faster than 18a(19a), which, in turn, eliminates
faster than the trans isomer 18c(19c). Think about it.
