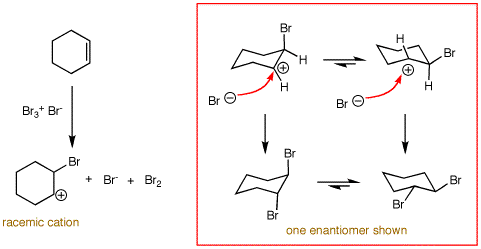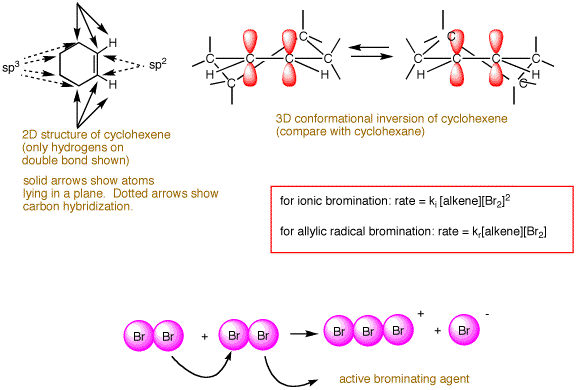

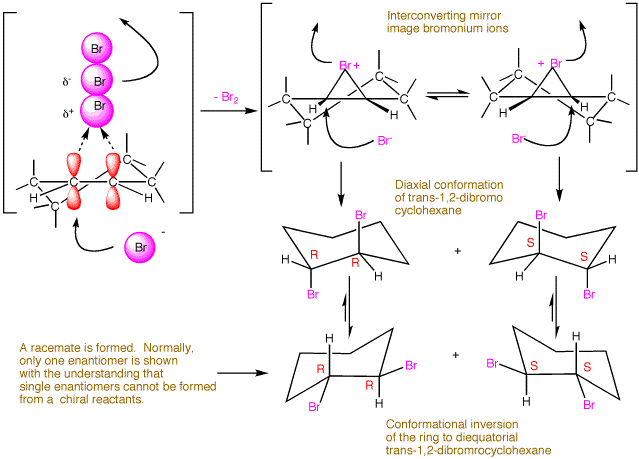
The kinetics of the reaction follow the rate law: RATE = ki[alkene][X2]2. The rate limiting step involves two molecules of bromine and only one of alkene even though the stoichiometry of the reaction is 1:1. Two molecules of bromine form the species Br3+ Br-. The cationic tribromide is the actual brominating agent. The kinetics for the free radical allylic bromination of an alkene follow the rate law RATE = kr[alkene][X2]. This is why free radical bromination occurs at low concentations of bromine where it is difficult for two molecules of bromine to form the bromonium bromide ion species. The nucleophilic displacement by bromide on the cyclic bromonium species occurs in a conformation that leads initially to the diaxial conformation of the 1,2-dibromide. Because the cyclic bromonium species is rapidly interconverting chairlike conformations, both enantiomers of the dibromide are formed although only one is shown. [Continued, scroll down.]
The astute student should be asking, "How
do you know that the bromonium ion doesn't open first, like
an SN1 reaction, form a secondary cation, and
then add bromide ion?" This argument might be supported by
saying that bromide ion likes to approach the cation for
steric reasons on the side of the ring that is remote from
the intact, bulky bromine atom. This hypothesis is
illustrated on the right. This argument may be countered by
examining the results obtained from the bromination of
(E)- and (Z)-2-butene. The (E)-isomer
affords meso-2,3-dibromobutane while the (Z)-isomer
produces racemic 2,3-dibromobutane. Such a reaction, in
which each of two stereoisomers produces a single, different
stereoisomer, is said to be stereospecific. Let's
test these observations and see how they stand up to the two
reaction mechanisms: bromonium ion vs. secondary
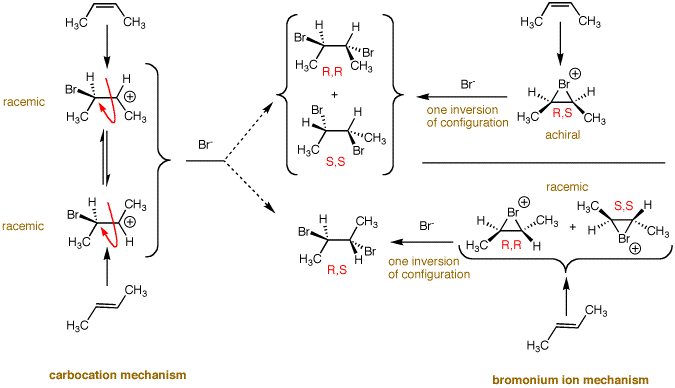
carbocation. In the diagram on the left, the left side
has the carbocation mechanism. Either 2-butene will form a
racemic mixture of cations. These cations are identical
because there is free rotation about the 2,3 C-C bond. The
cation loses all "memory" of where it came from. Thus, the
two 2-butene stereoisomers would be expected to form the
same compound, racemic or meso-2,3-dibromobutane, or an
identical mixture of the two. But this is not what is
observed! The reaction, as noted above, is stereospecific.
The bromonium ion mechanism explains the results. This mechanism applies not only to
symmetrically substituted alkenes but unsymmetrically
substituted ones as well. (E)- and
(Z)-3-methyl-3-hexene each gives a single racemate of
the two possible diastereomers of
3,4-dibromo-3-methylhexane. The mechanism applies to other dihalogen
additions as well as halohydrin formation.