Six π- Molecular Orbitals
(MO's) of Benzene
How to Manipulate Jmol Structures or Click on the Jmol logo.
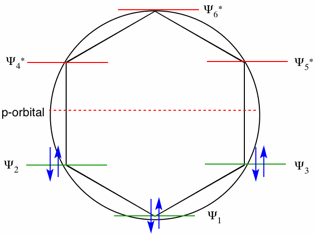
The diagram below is a Frost-Hückel circle mnemonic. A polygon representing the ring system, in this case a regular hexagon for benzene, is inscribed in a circle with a vertex at the bottom. The energy levels of the six-π-MO's are placed at each vertex. The energy of an isolated p-orbital is taken as passing through the center of the circle. From the geometry of a regular hexagon, there should be equal separation of the three energy levels. Compare with the energies on the right. There are three bonding MO's and three anti-bonding MO's. MO's Ψ2 and Ψ3 are degenerate (same energy) bonding orbitals while Ψ4* and Ψ5* are degenerate anti-bonding orbitals.
|
|