How to Calculate Percent Incorporation of an Isotope
 |
 |
|---|
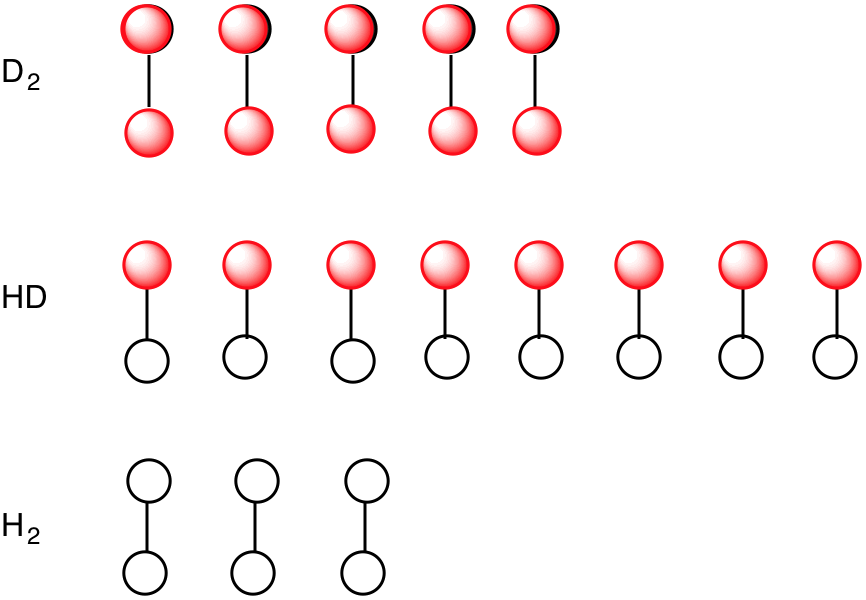
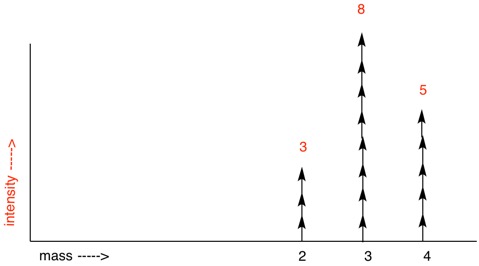
Consider the group of three isotopically labelled molecules on the left above: H2 (mass = 2), HD (mass = 3) and D2 (mass = 4). The open circles are hydrogens and the red circles are deuterium atoms. If asked, what is the percentage of deuterium atoms in these 16 molecules (32 atoms), all one needs to do is count the number of deuterium atoms (18) and divide by 32, i.e. 56.25%. While counting atoms or molecules directly is not an option, a mass spectrometer can accomplish the same task. A mass spectrum run at low voltage to minimize fragmentation would display three masses (intensity) of 2 (3), 3 (8) and 4 (5) (simulated spectrum on the upper right). The intensities in parentheses are in the same ratio as the number of molecules. The number of hydrogens/deuteria can be determined by using the peak intensity as a weighted adjustment factor (below). Multiplying #H and #D by the peak intensity gives #Hadj and #Dadj, respectively. Adding the #Hadj's and #Dadj's gives 14 hydrogens and 18 deuteria. This method permits the desired computation. Now return to perdeuteriocyclopentadecyne.
| #H | #D | peak intensity (# molecules) | #Hadj | #Dadj |
|
|---|---|---|---|---|---|
D2 |
0 | 2 | 5 | 0 | 10 |
| HD | 1 | 1 | 8 | 8 | 8 |
| H2 | 2 | 0 | 3 | 6 | 0 |
| Sum ----> | 14 | 18 |