On the Mechanism of Osazone Formation
Index:
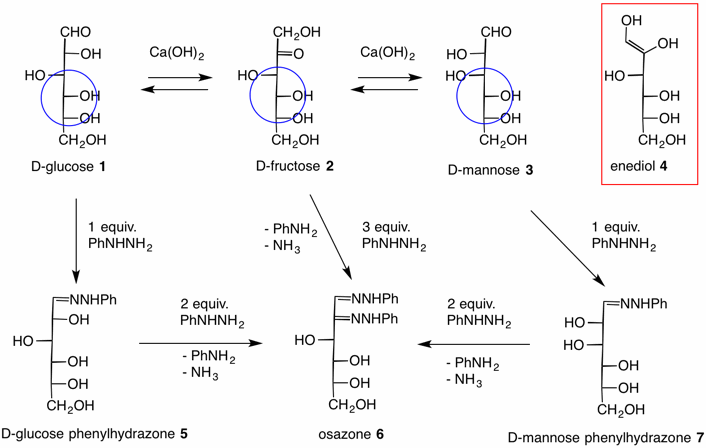
In 1875 Emil Fischer prepared phenylhydrazine (PhNHNH2) by the reduction of a phenyldiazonium salt. Years later (1891) this compound proved invaluable during his studies on the stereochemistry of glucose. The two aldohexoses, D-glucose 1 and D-mannose 3, and the D-ketohexose, D-fructose 2, are interconvertible with one another in the presence of calcium hydroxide without altering the stereochemistry at C3, C4 and C5 (atoms within the blue circles). The intermediate for this transformation is the enediol 4, which is formed via enolization. The process is known as the Lobry de Bruyn - Alberda van Eckstein rearrangement. Under mild conditions both D-glucose and D-mannose form their respective phenylhydrazones 5 and 7 (as does D-fructose which is not shown). Upon treating the phenylhydrazones with two additional equivalents of phenylhydrazine, all three hexoses are converted to the same osazone 6. Notice that one of the equivalents of phenylhydrazine is converted into aniline (PhNH2) and ammonia (NH3). This is a reduction of phenylhydrazine. The osazone is an oxidation product. Effectively, the C2 hydroxyl group of the phenylhydrazones has been oxidized to a ketone level removing the C2 stereochemistry. In addition the C1 hydroxyl of the fructose phenylhydrazone is oxidized to the level of an aldehyde. The question remains, how does this process occur? While the mechanism of the phenylhydrazone formation is a straightforward addition of phenylhydrazine to a carbonyl group with concommitent elimination of water, the osazone formation has been formulated by several mechanisms.

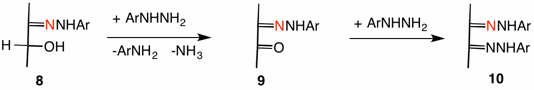
Emil Fischer's proposed a mechanism invoking initial formation of a phenylhydrazone 8 (Ar = Ph, ignore the red nitrogen) from an α-hydroxy aldehyde or α-hydroxy ketone. In an unspecified manner the second and third equivalents of phenylhydrazine effected oxidation of the secondary alcohol (structure 9) --- note the formation of the reduction products aniline and ammonia --- and derivatization to form the osazone 10. While this mechanism may be correct, the lack of evidence for the transformation 8 ---> 9 leaves Fischer's proposal as part speculation.
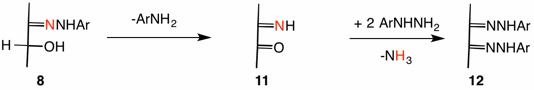
Weygand proposed two possible mechanisms based upon hydrogen isotope studies, both of which were claimed to be operable. Mechanism A has the phenylhydrazone 8 (Ar = Ph, ignore the red nitrogen) break the N-N bond to form aniline (reduction) and oxidize the alcohol in the same operation. The resulting α-iminoketone (α-iminoaldehyde) 11 is subsequently derivativized with phenylhydrazine to liberate ammonia, the osazone 12 and, of course, water.
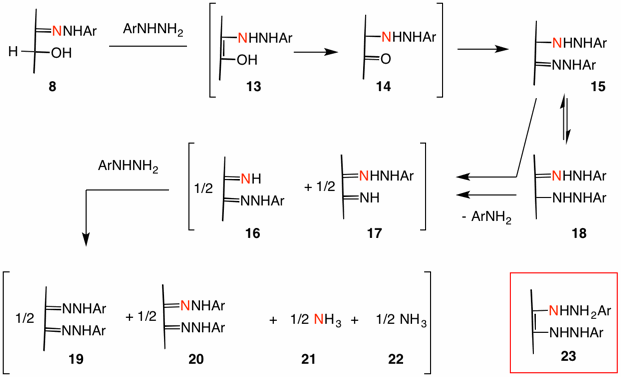
Weygand's second mechanism incorporates a second equivalent of phenylhydrazine into hydrazone 8 (Ar = Ph, ignore the red nitrogen) prior to oxidation. This process occurs via enolization and tautomerization --- structures 13 and 14 --- resulting in phenylhydrazone 15. This osazone can undergo tautomerization to constitutional isomer 18 via intermediate 23. The transformation 8 ---> 14 is an AmadoriOxidative loss of aniline from isomers 15 and 18 afford α-iminohydrazones 16 and 17, respectively. Exchange of phenylhydrazine with the imine results in the osazone 19/20 and ammonia 21/22. That is, 16 ---> 19 + 21; 17 ---> 20 + 22. [Note: The ratios 15/18, 17/16, 20/19 and 21/22 need not be precisely 50/50. ]
Shemyakin devised experiments designed to distinguish among the three proposed mechanisms. He utilized p-nitrophenylhydrazine as the derivatizing agent with the β-nitrogen labeled with 15N (p-O2NC6H4NHNH2) in the p-nitrophenylhydrazone 8. First, Shemyakin demonstrated that unlabeled p-nitrophenylhydrazine does not, to any extent, exchange with the labeled hydrazone 8, i.e., label dilution is not a problem. The additional two equivalents of p-nitrophenylhydrazine were unlabeled. The Fischer mechanism predicts that the labeled nitrogen shown in red in the "Fischer Mechanism" above, will be present in the osazone 10 but not in the aniline or ammonia. However, the labeled nitrogen was found in ammonia (NH3) and not in the osazone nor in aniline. This result clearly eliminated the Fischer mechanism from contention.
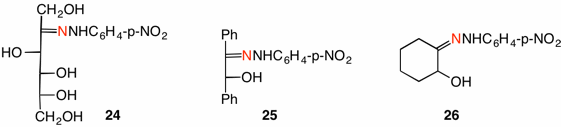
Both of the Weygand mechanisms predict that the labeled nitrogen will appear in ammonia. The difference is that mechanism A predicts all of the label is in ammonia and none in the osazone 12. On the other hand, mechanism B predicts half of the label in ammonia (21/22) and half in the osazone 19/20. The p-nitrophenylhydrazones of D-fructose (24), benzoin (25) and α-hydroxycyclohexanone (26) were prepared (40% labeled) and subjected to osazone formation with unlabeled p-nitrophenylhydrazine. In all three cases the ammonia collected was greater than 50% of the 40% label in 24-26. In addition, unreacted hydrazones 24-26 had the same percentage of label as they did before the reaction (no hydrazine exchange). These data support Weygand's mechanism A with little, if any, contribution from mechanism B.
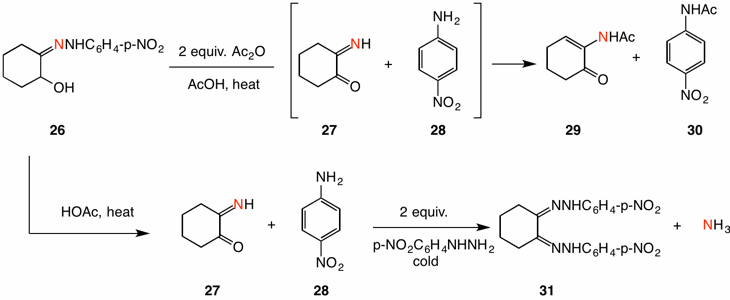
Additional experiments further confirmed these results by trapping the intermediates in the reaction prior to the intervention of unlabeled p-nitrophenylhydrazine. First, upon heating hydrazone 26 in acetic acid in the presence of 2 equivalents of acetic anhydride, intermediates α-iminocyclohexanone (27) and p-nitroaniline (28) were trapped by acetic anhydride as α-N-acetylcyclohexenone (29) and p-nitroacetanilide (30), respectively. Secondly, when hydrazone 26 was heated in acetic acid, cooled and treated with two equivalents of p-nitrophenylhydrazine in the cold, osazone 31 and p-nitroaniline (28) were isolated free of label along with labeled ammonia
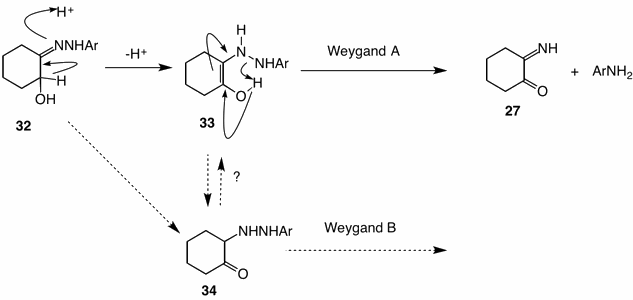
The mechanism for this redox step is formulated below. Enolization of hydrazone 32 occurs to afford the enol 33. Whether the collapse of the enol 33 is concerted as shown, or involves initial proton transfer to nitrogen (β-elimination pathway) is debatable. For the Weygand B mechanism to be inoperable either collapse of enol 33 to α-iminocyclohexanone (27) must be faster than ketonization to 34, i.e., ketone 34 is not formed, or the reaction of a phenylhydrazine (Weygand B) with ketone 34 must be slower than pathway 33 ---> 27 (Weygand A).
F. E. Ziegler, April 2016