Ester 7:
Problem:
How would you efficiently prepare monoester 2 from succinic acid 1?
Solution:
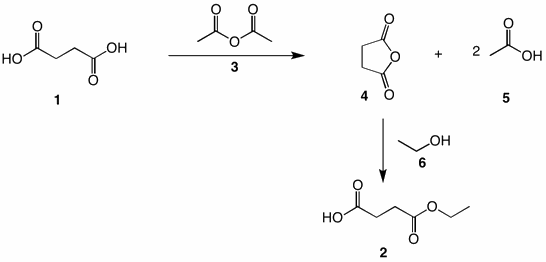
Acetic anhydride (3) dehydrates succinic acid (1) to form succinic anhydride (4) and two equivalents of acetic acid (5). Essentially, water is removed from succinic acid and added to acetic anhydride. Treatment of succinic anhydride with excess ethanol (no strong acid catalyst) affords the half acid ethyl ester 2. However, as the acid 2 accumulates, it can act as a catalyst. Fischer esterification of succinic acid with only one equivalent of ethanol (6) is expected to give a near statistical mixture of 1, 2 and diethyl succinate (1:2:1). The same is true of esterification with diazoethane. What is the mechanism for the conversion of succinic acid to its anhydride? Click here.