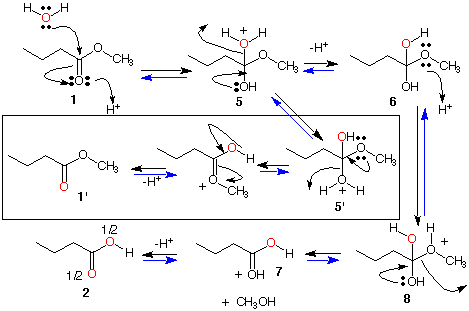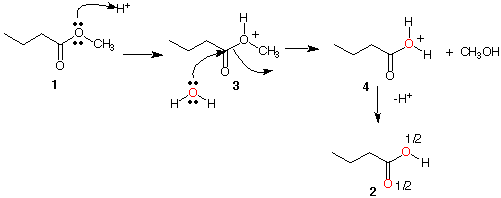
Ester 1:
Solution: O18 is designated by O.
Mechanism A: This mechanism accounts for the introduction of O18 into the acid 2 but not into the starting ester 1. Moreover, protonation as shown in 3 is less likely than protonation on the ester carbonyl oxygen. In the case of 3, the partial positve charge on the carbonyl carbon inhibits an adjacent positive charge on oxygen. In the same vain, resonance causes the oxygen carbonyl to have a higher negative charge and, therefore, be more susceptible toward protonation. See Mechanism B below.

Mechanism B; This mechanism not only accounts for the incorporation of O18 into acid 2 but also into ester 1. Reprotonation of tetrahedral intermediate 5 as 5' can occur. Reversal of the step 1 ---> 5 via the route 5' ---> 1' installs O18 into the carbonyl oxygen of ester 1. In fact, every step is reversible and is so indicated by the second arrow <-------. Why is the label in the acid at both locations in the carboxylic acid 2?