Aldehyde 8:
Problem

Solution
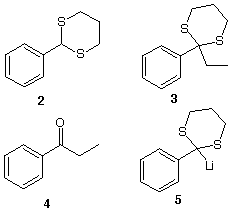
This sequence of reactions provides a method for
converting an aldehyde 1 (benzaldehyde) in to a
ketone 4 (ethyl phenyl ketone, propiophenone) via the
dithiane 2 (2-phenyl-1,3-dithiane). The reaction can
also be applied to aliphatic aldehydes. Because the polarity
of the carbonyl carbon is positive, it is difficult to
generate the anion of an aldehyde by deprotonation to form
an acyl anion. This reversal of polarity is known as
umpolung (Ger., pole reversal). The hydrogen at the
2-position of 2 has a pKa = ~ 29 (higher for
aliphatic derivatives. Both aromatic and aliphatic dithianes
are readily deprotonated by n-butyllithium. In this
instance, anion 5 is formed. What is the approximate
pKa of n-butyllithium? Here
is a pKa
table. Here is an
answer. Anion 5 forms 3 by
what type of
reaction with ethyl
iodide? Hydrolysis of the alkylated dithiane 3
liberates ketone 4. The mechanism for the formation
of dithiane 2 is one that you should be able to
handle if you known how acetals are formed. If not, review
this concept with the solution to aldehyde
#2. A more traditional route from aldehyde 2 to
ketone 4 is accomplished by the addition of ethyl
magnesium Grignard to 2 followed by oxidation of the
intermediate alcohol to ketone 4. Both of these
routes start with the same reactant and end with the same
product. Therefore, the total electron change in both routes
must be the same. Verify.