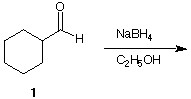
Aldehyde 3:
Problem
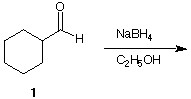
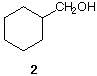
Solution
 Sodium borohydride (NaBH4) is a reducing
agent that is reasonably stable in ethanol and water. Methanol can be
used as a solvent but it reacts faster with NaBH4 than does
ethanol or water. NaBH4
reduces aldehydes to primary
alcohols and ketones to secondary alcohols. Each of the the four
hydride of NaBH4 is available for reduction. In this instance,
cyclohexylmethanol 2 is the product of reduction of
cyclohexanecarboxaldehyde
1. What other reducing agent is
available for the reaction ?
Sodium borohydride (NaBH4) is a reducing
agent that is reasonably stable in ethanol and water. Methanol can be
used as a solvent but it reacts faster with NaBH4 than does
ethanol or water. NaBH4
reduces aldehydes to primary
alcohols and ketones to secondary alcohols. Each of the the four
hydride of NaBH4 is available for reduction. In this instance,
cyclohexylmethanol 2 is the product of reduction of
cyclohexanecarboxaldehyde
1. What other reducing agent is
available for the reaction ?