On the Relative Acidity of Hydrohalic Acids
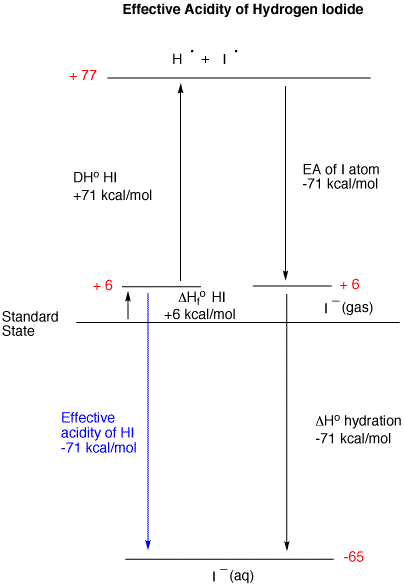
The acidity of the series of hydrohalic acids, HF, HCl, HBr and HI increases from HF through HI, a fact that seems counterintuitive given that fluorine is the most electronegative -- and iodine the least -- of the four halides. Several factors come into play including heat of formation of the hydrohalides (ΔHfo), bond dissociation energy (BDE), electron affinity (EA) and heat of hydration. To evaluate the relative acidity of this series, the two extreme cases will be considered first: HF and HI. The first step in the analysis is to consider the stability of the two hydrohalides. Clearly, the stability of HF is greater than HI as witnessed by their respective heats of formation, -65 and +6 kcal/mole. The bond dissociation energy of HF (+136 kcal/mol) is much greater than that of HI (+71 kcal/mole). These two factors place the atoms hydrogen and fluorine 71 kcal/mol above the Standard State while the hydrogen atom and iodine atom are 77 kcal/mol above the Standard State. Since all four hydrohalides form a hydrogen atom, we need not track its fate because it will be the same for all four. We seek only the relative acidity of the hydrohalides, not the absolute value. Continued below.
 |
 |
|---|
At this juncture we have a fluorine and iodine atom in the gas phase. Since an aqueous acid has a negatively charged halide solvated by water, we must get these gas phase atoms into an aqueous environment.. The electron affinity of fluorine atom to produce gas phase fluoride is -79 kcal/mol; for an iodine atom reduced to iodide, the value is -71 kcal/mol. Because fluoride is more basic than iodide; its heat of hydration is much more exothermic (-121 kcal/mol) than the hydration of iodide (-71 kcal/mol). It is not legitimate to compare the energy of aqueous fluoride (-129 kcal/mol) with aqueous iodide (-65 kcal/mol) and assume, owing to the more negative value of aqueous fluoride, that hydrofluoric acid is more acidic than hydroiodic acid. What is more important is where you begin and where you end. The effective comparison is HF ---> fluoride [aq. (-64 kcal/mol)] vs. HI ---> iodide [aq. (-71 kcal/mol)]. HI is a stronger acid than HF. The term "effective" is employed because the conversion of gaseous hydrogen atom to aqueous proton has been ignored in both analyses.
The chart below lists the data for the four hydrohalic acids in kcal/mol.
HX |
ΔHfo |
DHo |
ΔHfo + DHo |
E.A. |
ΔHo(hydration) |
Eff. Acidity |
|---|---|---|---|---|---|---|
HF |
-65 |
+136 |
+71 |
-79 |
-121 |
-64 |
HCl |
-22 |
+103 |
+81 |
-83 |
-87 |
-66 |
HBr |
-8.6 |
+87.5 |
+79 |
-77 |
-80 |
-69.5 |
HI |
+6 |
+71 |
+77 |
-71 |
-71 |
-71 |