Organic Chemistry
Chem 220a
Chapter 5
Due: Monday, October 4, 1999

Michelangelo-The Creation of Man
1508-12 Sistine Chapel
|

Michelangelo-The Creation of Man 1508-12 Sistine Chapel |
1) Read the stereochemistry module in the StudyAids and do the exercises. There is no need to record answers on your homework. http://classes.yale.edu/chem220a/studyaids.html#Stereoisomers
|
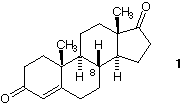
2) Assign R,S-configurations to the remaining stereogenic centers in androstendione (on the right). Why is C8 of the R-configuration? How many stereoisomers of this structure are possible (excluding double bond geometrical isomers)? |
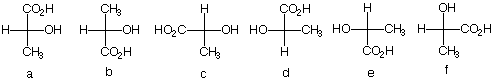
 |
4) (R)-(+)-Limonene, which appeared on exam 1, is reduced in the presence of two equivalents of H2 to a
mixture of two optically inactive compounds. How is the loss of optical activity possible? Illustrate and
explain. Draw (R)-(+)-limonene. How is it possible that (R)-limonene and (S)-lactic acid (#2 above) are
both dextrorotatory and yet have opposite R,S-configurations?
5) Two students measure the rotation a of a compound A. She uses a 0.028 M methanolic solution of A and
a 1 dm cell. The observed rotation a is +110.8o; the investigator concludes that A is dextrorotatory and
optically active. He uses the same cell (cleaned, of course) and a 0.091 M methanolic solution of A and
concludes that the compound is achiral or racemic because there is no observed rotation. Explain.
6) "Natural" tartaric acid is of the R, R-configuration and it has an [a]D = +12 o. What is the specific rotation
of the R, S-isomer? What is the specific rotation of a mixture that is a 2:1 mixture of R,S and R,R tartaric
acid, respectively?
|
7) Levorotatory a -pinene 1, a constituent of turpentine, has a specific rotation of 51.2 o. A sample of a-pinene contaminated with its enantiomer has an [a]D= -33.5 o. How much of each enantiomer is present in the mixture? What is the configuration of the two asymmetric carbons in 1? What is the configuration of the enantiomer of 1? The diastereomer of 1 (or its enantiomer) does not exist. Why? Justify your answer.
|
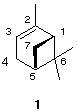 |
What is the principle involved in the resolution? Which enantiomer is in excess? What are the rotations of
the pure enantiomers?