1) Do the six problems in the Alkane
module of ORGO [Organic Reactions Go
Online] for practice. Work out the problem
BEFORE looking at the answer.
[http://classes.yale.edu/chem220a]
2) The radical chain reaction between ethane and I2
to form ethyl iodide does not occur yet the
corresponding reaction with Br2 is successful.
Explain and illustrate with the use of energy
diagrams and the heats of reaction for each
propagation step and for the overall reaction.
3) Why doesn't the radical chain reaction in #2 that forms ethyl bromide proceed as shown below? Illustrate and explain.
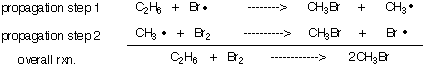
4) Draw the structures of the monochlorination
products of 2,2-dimethylbutane? What
percentage of each is formed by radical chain
chlorination of the alkane?
5) Why is the benzylic CH bond dissociation
energy in toluene (1) much lower that the value
for ethane. See pg. 142.
6) Provide 3D representations of the following species and describe in a word their respective geometries: allyl radical, tert-butyl cation, trimethylamine.