1) Name the following compound, C17H36. Click here for a static structure or here for a ChimeTM rotatable
presentation.] (Download the ChimeTM . How to manipulate structures.)
2) Draw the chair conformations for trans-1,4-bromochlorocyclohexane. What is the
energy of each of the chair conformations? What is the difference in energy between
the two conformations? What percentage of each conformation is present at 27oC?
3) DGo=0 for the difference in energy between the chair conformations of cis-1,4-
dimethylcyclohexane. Why? The difference in energy between the chair conformations
of cis-1,3-dimethylcyclohexane is very large. Why?
4) Draw the staggered and eclipsed conformations of 2-methylbutane about the C2-C3
bond. What is the energy of each conformation? Draw a graph similar to the one on
page 103.
5) Draw (line-angle formulas) the isomers of C6H14. Which one has the lowest boiling
point and why?
[https://classes.yale.edu/chem220a/studyaids/isomers/isom_intro/isomer.html]
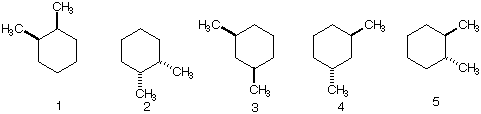
6) Which of the following dimethyl cyclohexanes are identical? How many different
constitutional isomers are there? Which one(s) have two equatorial methyl groups in
their most stable chair conformation? Which one(s) have one equatorial methyl group
in their most stable chair conformation?
