Chem 220a
Problem Set 10
Chapters 13
NMR Spectroscopy
Due: Monday, December 6, 1999
|
1) Of homotopic, enantiotopic, and diastereotopic
hydrogens, which one(s) have the same
chemical shift and which ones(s) have different
chemical shifts? Locate the three types of
protons in 1- and 2-bromobutane.
2) Draw a 1H NMR spectrum of 1-bromobutane having
the chemical shifts in the correct order.
3) Predict the number of signals in the decoupled
13C NMR spectrum of all the structural isomers of C5H11Br.
|
A spectrum recorded during the infancy of NMR.
http://www.nmr.varian.com/
|
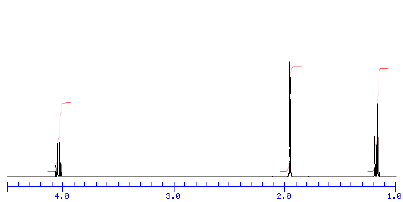
4) Compound A (C4H8O2) has the 1H NMR spectrum shown below.
When compound A reacts with an
excess of CH3MgBr, two compounds, B and C, are isolated. The 1H
NMR spectrum of B is shown on
the right in an old spectrum. One of the signals in this spectrum
disappears when D2O is added to the
sample. Compound C has only two singlets (9:1 ratio) in its 1H
NMR spectrum. The smaller singlet
disappears on addition of D2O to the sample. What are the
structures of A-C? Explain. [The signals in
the spectrum (reading from left to right) are a quartet, singlet,
and triplet.]
5) Compound A (C6H12O) reacts with NaBH4 to form B (C6H14O).
Treatment of B with H2SO4 at 150 oC
provides C (C6H12). Exposure of C to OsO4 (catalytic) and H2O2
affords D (C6H14O2). The reaction of
D with H2SO4 at 100 oC gives A. Compound A: 1H NMR d 2.2 (3H, s) and 1.1 (9H, s). [The chemical
shifts are estimated.] What are the structures of A-D? Explain.
[Notice that there is a two electron
reduction and a two electron oxidation in the cycle of A --> B
--> C --> D --> A.]
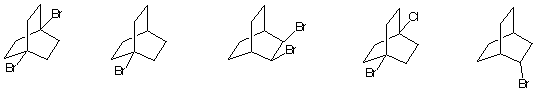
6) During free radical chlorination of A (C5H12) a dichloro
compound B was isolated. The 1H NMR
spectrum of B displayed two singlets in a 3:2 ratio while the
decoupled 13C NMR spectrum displayed
three singlets, one of which was a weak signal. What are the
structures involved? Explain.
7) Predict the number of singlets in the proton decoupled 13C NMR
spectrum of the following alkyl halides.
Illustrate.
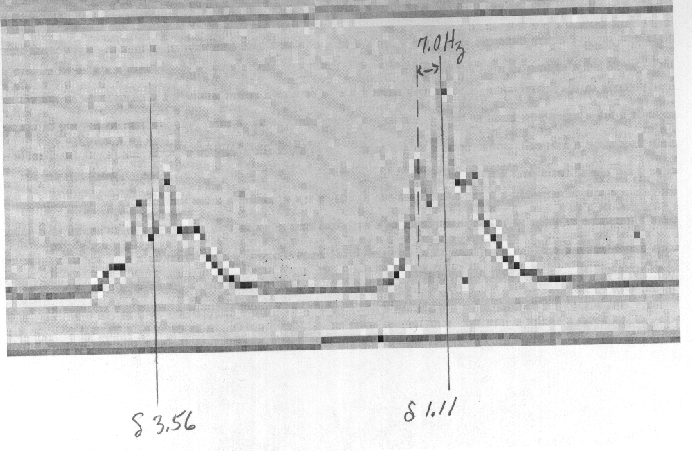
8) Estimate the frequency (MHz) of the 1H NMR spectrometer that
recorded the spectrum shown to the right
of problems 1 -3. The chemical shifts are d
1.11 for the triplet and d 3.56 for
the quartet. Assume the
value of J = 7.0 Hz. A blow-up of the necessary part of the
spectrum is shown below.