Do Alkyl Halides 1 and 5 in ORGO
1) Carbonic acid (H2CO3) has the following ionization constants: K1=4.3 x 10-7 and K2 = 5.6 x 10-11. If phenol is dissolved in ether, phenol can be extracted from the ether phase (ether and water are immisible) with aqueous NaOH but not with aqueous NaHCO3. Explain.
2) Compound A (C5H10O) reacts with ethyl magnesium bromide to form B (C7H16O). B reacts with sodium to liberate hydrogen. Treatment of B with H2SO4 forms only C (C7H14). C reacts with Br2 to form D (C7H14Br2). Ozonolysis of C produces A and E (C2H4O). What are the structures of A-E and explain their formation?
3) When ethyl benzoate is treated with one equivalent of methyl magnesium iodide, 2-phenyl-2-propanol is formed in ~50% yield and ethyl benzoate is recovered. There is little if any of the expected acetophenone. Explain.
4) When (R)-2-bromooctane reacts with lithium di-n-butylcuprate, (R)-5-methylundecane is formed. What is the mechanism of the reaction?
5) When 5-bromo-1-pentanol reacts with magnesium in ether followed by treatment with water, 1-pentanol is formed. If D2O is used instead of H2O, no carbon bound deuterium is found in the 1-pentanol. Explain.
6) Devise a series of reactions to convert 2-methyl-2-butanol into 3-methyl-2-butanol.
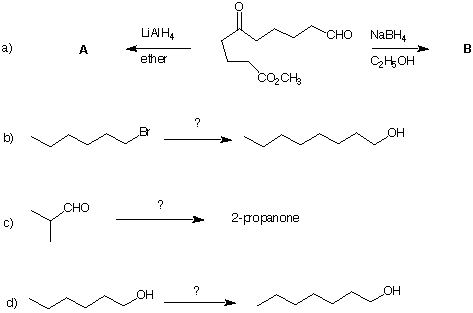
7) Provide the products or reagents in each of the following problems. The last three questions require more than one step to effect the transformation. Include the compounds that are formed along the way.
