This problem set covers chapters 7 and 8. It is due in two weeks.
Chapter 7:
1) Treatment of (3R, 4R)-3-bromo-3,4-dimethylhexane A with aqueous sodium hydroxide gives a
tetrasubstituted alkene B and an (E)-trisubstituted alkene C. Why is (E)-C favored over (Z)-C? Does the
racemate give the same products? Explain. What stereoisomers of B and C are formed from a
diastereomer of A?
2) Construct a diagram (similar to pg. 312) that interrelates the heats of formation and the heats of
hydrogenation of (E)-2-hexene, (Z)-2-hexene and n-hexane. Provide numerical values. [Use Study Aids]
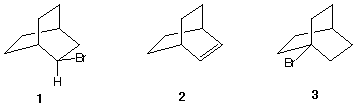
3) Treatment of bromide 1 with t-C4H9OK gives only bicyclic alkene 2. Bromide 3 is unreactive under the same conditions. Explain. Provide an accurate mechanism for the formation of 2 from 1.

4) (E)-Cyclooctene is capable of resolution but (E)-cyclododecene is not. Explain. Given that Pt can form complexes with alkenes, how would you use Pasteur's method (conceptually) to resolve (E)-cyclooctene into its enantiomers?
5) Predict the stereochemistry of the product of the reaction of (±)-1,2-dibromo-1,2-diphenylethane (1) with t-C4H9OK. Explain. What is the stereochemistry of the product from 1 when iodide ion is the reagent?
Chapter 8:
Study the Alkene module in ORGO first.
6) What alkene forms meso-3,4-dibromohexane upon bromination? Defend your answer with a mechanism.
7) What is the stereochemistry of the product derived from the peracid epoxidation of (Z)-4-octene? Explain and illustrate.
8) When (4R, 5R)-5-chloro-4-octanol (C8H17ClO) is treated with t-C4H9OK in t-C4H9OH, compound A (C8H16O) is formed? What is the structure of A? What is its optical rotation? Explain. [Hint: Think Williamson ether synthesis.]
9) Compound A, C6H12, reacts with OsO4 to form B. A forms only acetone on ozonolysis. Exposure of B to H2SO4 forms t-butyl methyl ketone (3,3-dimethyl-2-butanone) C. What are the structures of A, B and C? Explain and provide mechanisms for the transformations.
10) An optically active compound A, C9H15Cl, reacts with water to give (±)-B, C9H16O. Ozonolysis of B followed by treatment of the reaction mixture with dimethylsulfide leads to C, a ketoaldehyde (C9H16O3; 3-ethyl-3-hydroxy-6-oxoheptanal). Deduce the structures of A, B and C. Show your reasoning. [Help: see Degrees of Unsaturation in the Study Aids or your text to help in determining the number of rings and/or multiple bonds. Oxo is the C=O of a ketone; al is an aldehyde. Numbering starts at the aldehyde carbon.]
11) When optically active compound (R)-A, C6H13Br, is heated in ethanol, two products, B (C8H18O, racemic) and C (C6H12), are formed, neither of which is optically active. Ozonolysis of C followed by dimethyl sulfide reduction gives only 2-propanone. Deduce the structures of A, B and C. Show your reasoning. [Help: Info from chapter 5 is used here.]
12) Hydroboration and alkaline peroxide oxidation of (E)-3-methyl-3-heptene leads to an alcohol. How many stereogenic centers are present? How many diastereomers are present? Is the product racemic or not? What is the alcohol's structure and stereochemistry?