1) Do Alkyl Halides 2 and 3 and Ethers 1 in ORGO.
2) In the elimination reaction of 1-bromo-1-methylcyclohexane with RONa, the ratio of methylenecyclohexene to 1-methylcyclohexene is smaller when R = CH3 than when R = t-C4H9. Explain (see pg. 271). Is the reaction E1 or E2? Explain.
3) When a mixture of (R)-2-bromobutane and ethanethiol (1 equivalent each) is added to 1 equivalent of NaOCH3 in methanol, (S)-sec-butylethylsulfide is formed. Explain. [Hint: Think pKa and nucleophilicity.]
4) When meso-2,5-dibromohexane is treated with aqueous KOH, one of the products of the reaction is d,l-2,5-dimethyltetrahydrofuran (C6H12O) ? [Help: pg.597. Think Williamson ether synthesis]. Provide a mechanism.
5) The reaction of a mixture of cis-4-tert-butyl-1-bromocyclohexane and trans-4-tert-butyl-1-bromocyclohexane (1 molar equivalent each) with 1 molar equivalent of tert-C4H9OK provides 4-tert-butyl-1-cyclohexene and recovered trans isomer. Explain. [Think about chair conformations and the mechanism of the reaction.]
6) Treatment of tert-butyl bromide-d6 [(CH3)CBr(CD3)2] with aqueous NaOH produces more CH2=C(CD3)2 than CD2=C(CH3)(CD3). Explain
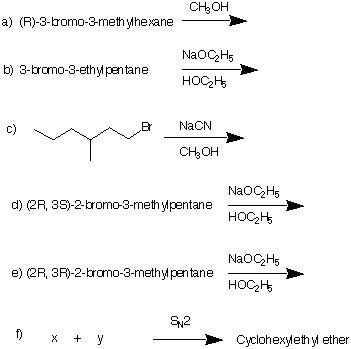
7) Predict the major product in each of the following reactions. Explain. Pay attention to mechanism ans stereochemistry.
