1) Draw 3D (sawhorse) and Fischer projections for (2R, 3S) - 2-bromo-3-methylpentane (1). Name the
enantiomer of this compound. Name the diastereomers of compound 1.
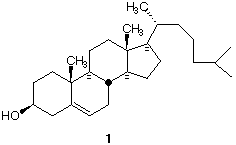
2) Structure 1 (C27H46O) represents the absolute stereochemistry of cholesterol. There are 28 non-geometric
(29 including the double bond) stereoisomers of cholesterol, one of which is its enantiomer. Assign R,S
configurations to each of the stereogenic centers in 1.

3) Two enantiomers of equal and opposite rotation ([a]D = 160o) are inadvertently mixed in the laboratory.
The rotation of the mixture shows [a]D= -96o. What is the optical purity of the mixture? What % of each
enantiomer is present in the mixture?
4) A partially racemized compound ( [a]D= 48o) is analyzed by chromatography using a chiral support and is
shown to be a 2:3 mixture. Why is this technique possible? What is the rotation of the pure enantiomers?
Which one is the minor isomer? What is the ee?
5) Two chemists measure the specific rotation of an organic compound. He measures a rotation of [a]D= 0o
while she obtains a non-zero value? What happened? How can the discrepancy be resolved?
6) When an optically active compound A, C8H12, is hydrogenated in the presence of a catalyst, cis and trans-
1,4-dimethylcyclohexane are formed. How many molar equivalents of hydrogen are absorbed in this
reaction? What structures are possible for A? Which ones are not possible? What other piece of
information is not known about A?
7) An optically active compound A has the formula C5H11Br. How many degrees of unsaturation are present?
(See Degrees of Unsaturation in the Study Aids.). A reacts with (n-C4H9)3SnH, a reducing agent, to give
B, C5H12, and (n-C4H9)3SnBr. Is B optically active? Is there sufficient information here to deduce the
structures of A and B (not the absolute stereochemistry of A)? If so, what are the structures of A and B;
if not, what structures fit A and B? Write a generic (RBr) mechanism for the reduction. [Check alkyl halides in ORGO]