1) Draw Lewis structures for C2H6, C2H4, HOCH2CH2CHO and CH3CH2CN
2) Draw Lewis structures for all the isomers of C3H9N.
3) Provide an example of a neutral and negatively charged resonance structure.
4) A compound has a pKa = 4.76. What is the value of Ka? In what form will this compound
exist in aqueous NaOH?
5) Is KNH2 more stable in n-pentane or water? Explain.
6) Acrylonitrile, CH2=CHCN (1), is used in the formation of polymers. Draw an orbital picture
of 1 and label the types of bonds and the hybridization of the carbon atoms.
7) Which of the following molecules have net dipole moments? Explain briefly.
![]()
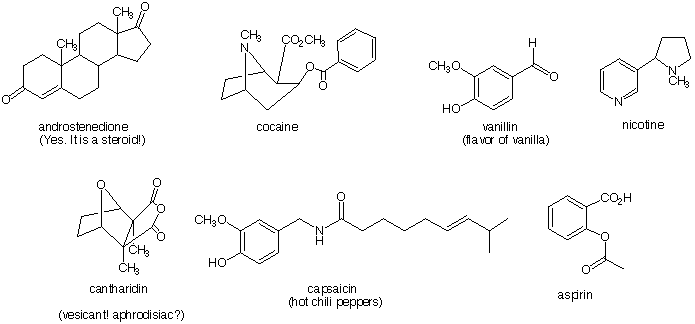
8) Locate and name the functional groups in the molecules shown below.