Problem Set 7
Chem 220
Due : Monday, October 27, 1997
Problems on Chapter 10
1. There are two different bonds that can be formed in the synthesis of ethyl isopropyl ether by the method of Williamson. Only one of the two routes will lead to the desired product. Explain. [Check with ORGO if you have difficulty]
2. How much K2Cr2O7 is required to oxidize 1.02 g of cyclohexanol?
3. Compound (S)-A (C5H12O2) is optically active. When A reacts with Na metal it forms B (C5H11O2Na). Acidification of B with aqueous HCl gives (S)-A back. Treatment of B with methyl iodide affords optically inactive C. What are the structures A-C and show your reasoning?
4. When PCC is added portionwise to n-butyl alcohol, compound A, C4H8O, and compound B, C8H16O2 are formed. Explain.
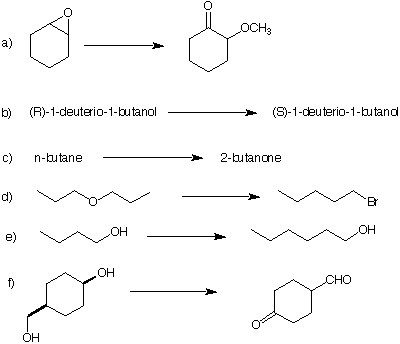
5. Provide the reagents and show mechanisms for each of the transformations, some of which may require more than one step.
