1. Which of the following compounds can and cannot exist as enantiomers?
Isobutyraldehyde, 3-pentanol, 2-pentanol, and 1-pentanol, Illustrate.
2. Draw (R)-bromochloroiodomethane, (S)-2-bromooctane, and (S)-3-hexanol.
Illustrate.
3. The (R)-enantiomer of a compound has a rotation of +60o. What is the optical
rotation of a 3:1 mixture (S:R) of the two enantiomers?
4. A sample of a mixture of enantiomers of a compound has an observed rotation of
-40o. If the absolute value of a pure enantiomer is 160, what is the percent of each
enantiomer in the mixture?
5. Draw all the stereoisomers of 2,3-dichlorobutane. Label them by the R,S method.
Which ones are enantiomers. What diastereomeric relationships exist? Which one is
incapable of resolution?
6. Enantiomeric excess (ee), i. e., the excess of one enantiomer over another, is defined
as the difference between the major and minor enantiomer divided by the sum of the
two. If the mole fraction of an R enantiomer is x, show that ee = 2x - 1. Show that
the equation holds for a racemate and a pure enantiomer.
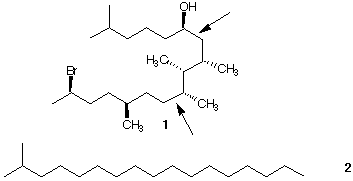
7. Give the R,S configurations for each of the stereogenic centers in structure 1.
Redraw structure 1 in template 2. [One way to think about this is to visualize rotation
about the two bonds (indicated by arrows) by 180o]. Check your R, S
configurations.
